Abstract
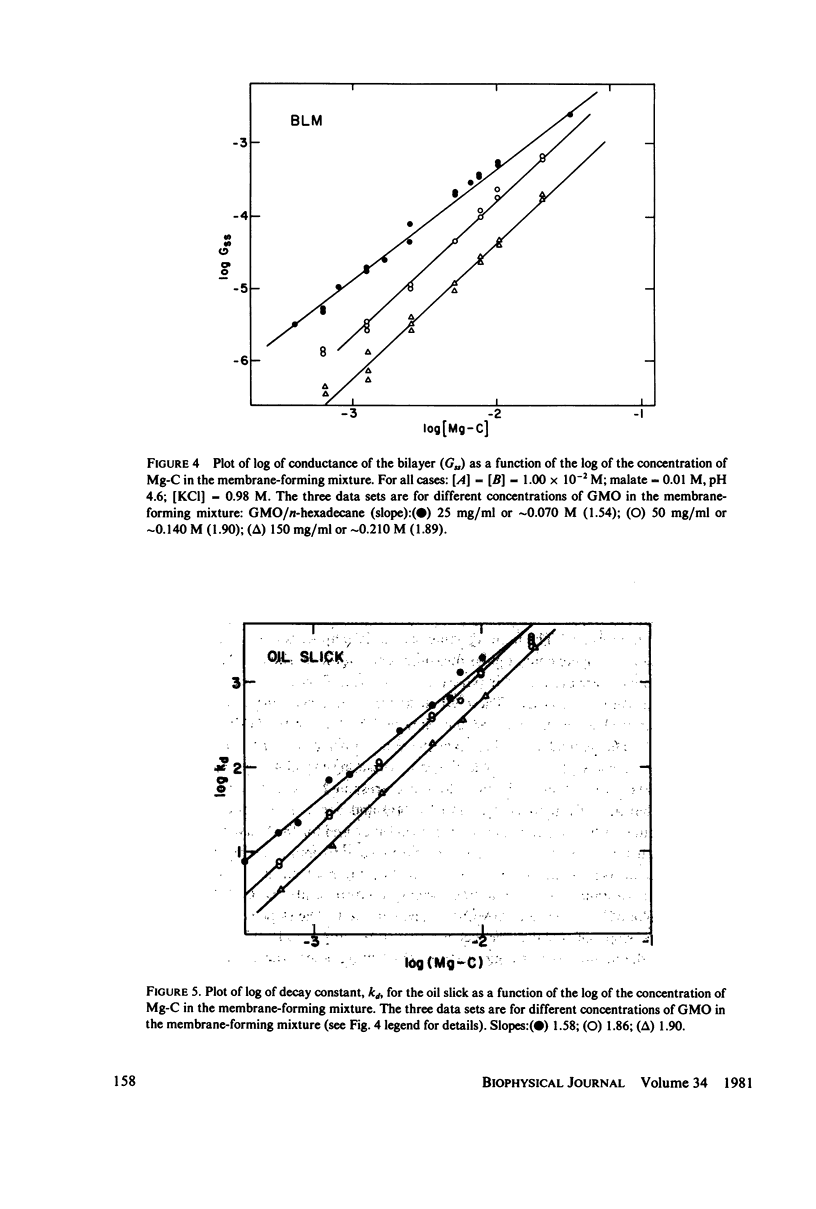
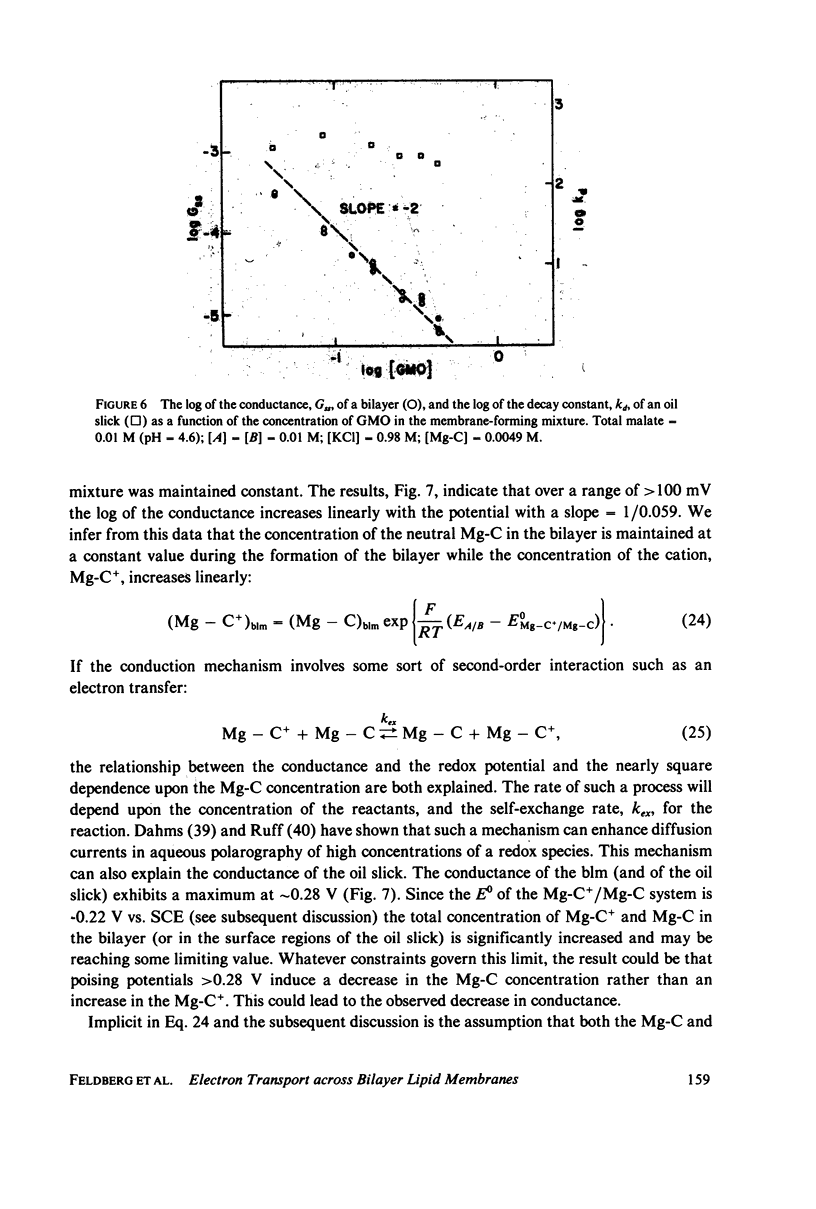
The transport of electrons across biological membranes is believed to play an important role in many biophenomena. Although there have been many examples of systems which may be transporting electrons across Mueller-Rudin bilayer lipid membranes (blm), none has been well characterized. The system we describe here comprises a glycerol monooleate blm containing a magnesium etiochlorin (Mg-C) separating two aqueous phases each containing ferricyanide, ferrocyanide, KCl, and a platinum electrode. The E0s for the Mg-C+/Mg-C and ferri-/ferrocyanide couples are 0.22 and 0.24 V vs. SCE. Thus the MG-C+/Mb-C system is easily poised by the ferri-/ferrocyanide system. When the potentials of the ferri-/ferrocyanide couples are different on each side of the blm we show that the open-circuit membrane potential nearly equals the difference between the redox potentials. This is unequivocal evidence that electrons are being transferred across the blm from one aqueous phase to the other. On the basis of these experiments we deduce that electron transport is the major charge transport mechanism. When redox potentials are the same on each side of the blm, the conductance of the membrane can be greater than 10(-3) S/cm2. The conductance is proportional to the second power of the concentration of Mg-C in the membrane-forming mixture. A number of additional experiments are described which attempt to elucidate the mechanism of electron transfer. We believe that our data are consistent with the idea of an electron-hopping mechanism in which the transmembrane electron transport occurs by a series of second-order electron transfers between membrane-bound electron donors (Mg-C) and acceptors (Mg-C+). Alternative explanations are presented.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Läuger P. Kinetic analysis of carrier-mediated ion transport by the charge-pulse technique. J Membr Biol. 1976 Jun 9;27(1-2):171–191. doi: 10.1007/BF01869135. [DOI] [PubMed] [Google Scholar]
- Berns D. S. Photosensitive bilayer membranes as model systems for photobiological processes. Photochem Photobiol. 1976 Aug;24(2):117–139. doi: 10.1111/j.1751-1097.1976.tb06807.x. [DOI] [PubMed] [Google Scholar]
- Ciani S. M., Eisenman G., Laprade R., Szabo G. Theoretical analysis of carrier-mediated electrical properties of bilayer membranes. Membranes. 1973;2:61–177. [PubMed] [Google Scholar]
- Feldberg S. W., Kissel G. Charge pulse studies of transport phenomena in bilayer membranes. I. Steady-state measurements of actin- and valinomycin-mediated transport in glycerol monooleate bilayers. J Membr Biol. 1975;20(3-4):269–300. doi: 10.1007/BF01870639. [DOI] [PubMed] [Google Scholar]
- Feldberg S. W., Nakadomari H. Charge ulse studies of transport phenomena in bilayer membranes. II. Detailed theory of steady-state behavior and application to valinomycin-mediated potassium transport. J Membr Biol. 1977 Feb 24;31(1-2):81–102. doi: 10.1007/BF01869400. [DOI] [PubMed] [Google Scholar]
- Finkelstein A. Weak-acid uncouplers of oxidative phosphorylation. Mechanism of action on thin lipid membranes. Biochim Biophys Acta. 1970 Apr 7;205(1):1–6. doi: 10.1016/0005-2728(70)90055-1. [DOI] [PubMed] [Google Scholar]
- Ford W. E., Otvos J. W., Calvin M. Photosensitized electron transport across lipid vesicle walls: quantum yield dependence on sensitizer concentration. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3590–3593. doi: 10.1073/pnas.76.8.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M., McLaughlin S. Complexes between uncouplers of oxidative phosphorylation. J Membr Biol. 1974;17(2):155–180. doi: 10.1007/BF01870177. [DOI] [PubMed] [Google Scholar]
- Hauska G. Plasto- and ubiquinone as translocators of electrons and protons through membranes: a facilitating role of the isoprenoid side chain. FEBS Lett. 1977 Jul 15;79(2):345–347. doi: 10.1016/0014-5793(77)80817-x. [DOI] [PubMed] [Google Scholar]
- Ilani A., Berns D. S. A theoretical model for electron transport through chlorophyll-containing bileaflet membranes. Biophysik. 1973 May 30;9(3):209–224. doi: 10.1007/BF01184686. [DOI] [PubMed] [Google Scholar]
- Kurihara K., Sukigara M., Toyoshima Y. Photoinduced charge separation in liposomes containing chlorophyll a. I. Photoreduction of copper(II) by potassium ascorbate through liposome bilayer containing purified chlorophyll a. Biochim Biophys Acta. 1979 Jul 10;547(1):117–126. doi: 10.1016/0005-2728(79)90100-2. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Mangel M., Berns D. S., Ilani A. Dependence of photosensitivity of bileaflet lipid membranes upon the chlorophyll and carotenoid content. J Membr Biol. 1975;20(1-2):171–180. doi: 10.1007/BF01870634. [DOI] [PubMed] [Google Scholar]
- Schönfeld M., Montal M., Feher G. Functional reconstitution of photosynthetic reaction centers in planar lipid bilayers. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6351–6355. doi: 10.1073/pnas.76.12.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien H. T. Electronic processes and photoelectric aspects of bilayer lipid membranes. Photochem Photobiol. 1976 Aug;24(2):97–116. doi: 10.1111/j.1751-1097.1976.tb06806.x. [DOI] [PubMed] [Google Scholar]
- Tien H. T. Electronic processes and photosensitization in bilayer lipid membranes. Photochem Photobiol. 1972 Oct;16(4):271–290. doi: 10.1111/j.1751-1097.1972.tb06298.x. [DOI] [PubMed] [Google Scholar]
- Wasser P. K., Fuhrhop J. H. The photooxygenation of metalloporphyrins and metallochlorins. Ann N Y Acad Sci. 1973;206:533–548. doi: 10.1111/j.1749-6632.1973.tb43235.x. [DOI] [PubMed] [Google Scholar]
- Young R. C., Feldberg S. W. Photoinitiated mediated transport of H3O+ and/or OH- across glycerol monooleate bilayers doped with magnesium octaethylporphyrin. Biophys J. 1979 Aug;27(2):237–255. doi: 10.1016/S0006-3495(79)85214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


