Abstract
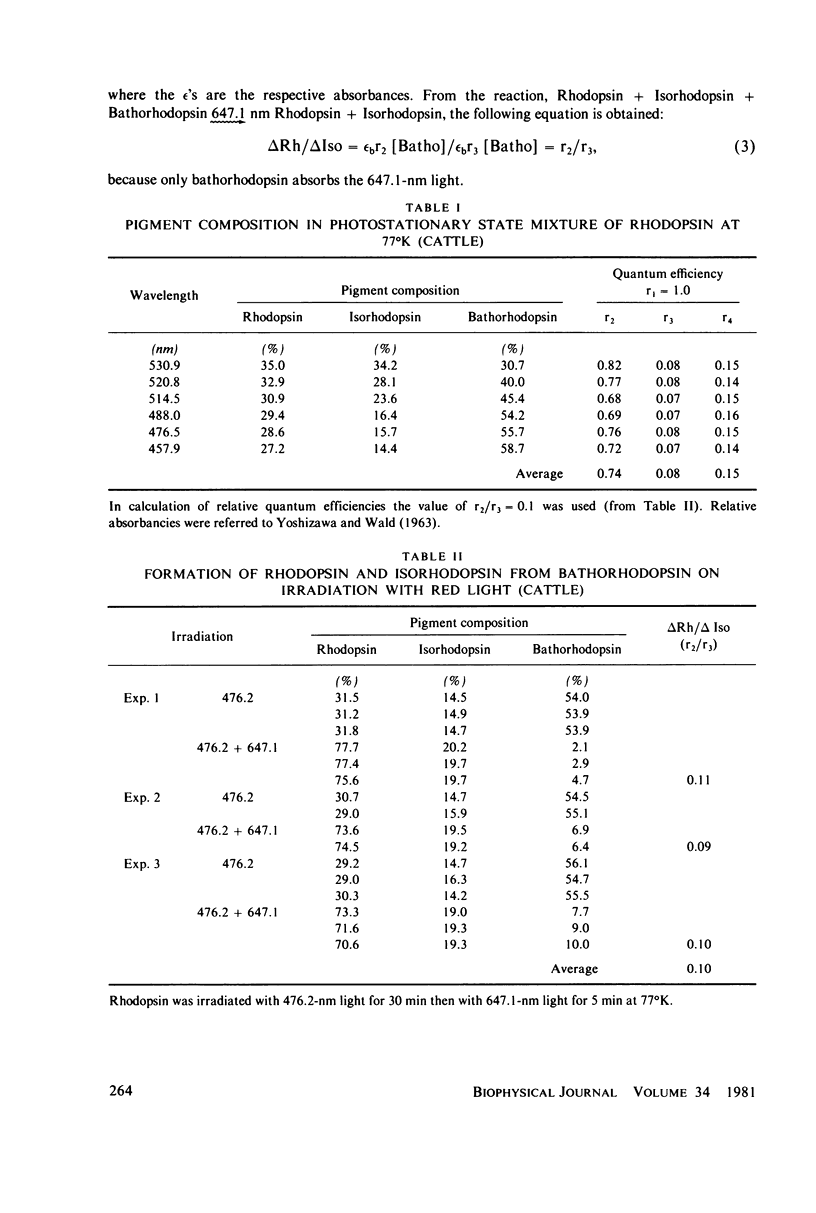
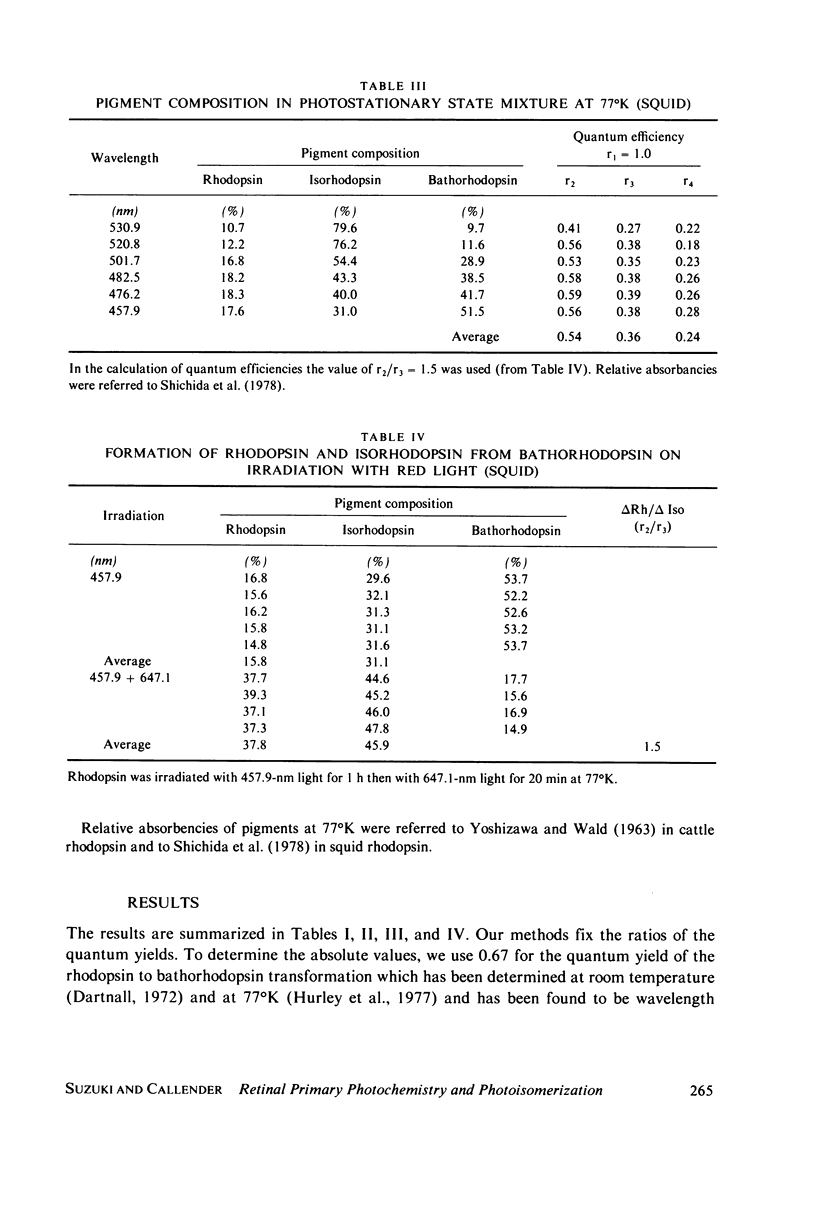
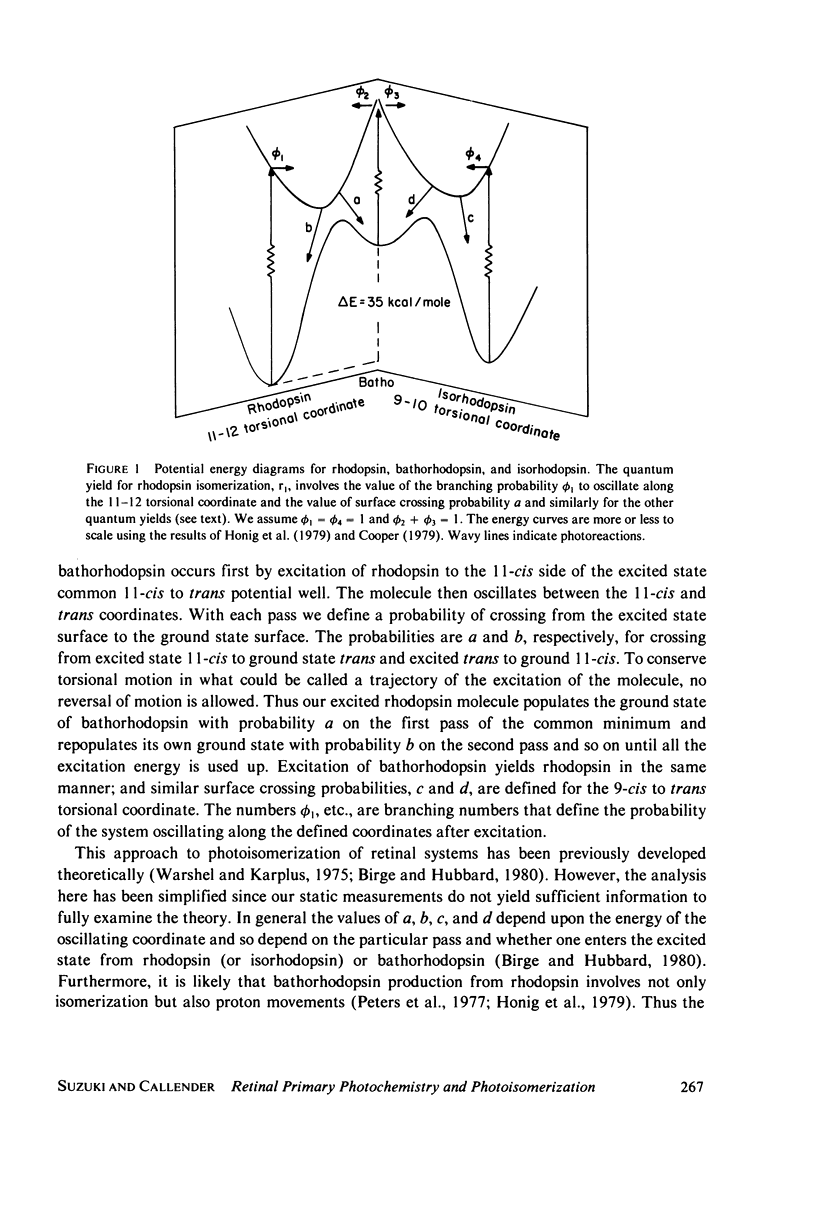
The relative quantum yields of the photoreactions Rhodopsin in equilibrium Bathorhodopsin in equilibrium Isorhodopsin over an extended wavelength region have been determined in cattle and squid rhodopsins at 77 degrees K. The quantum yields were found to be wavelength independent and unchanged for samples suspended in D2O. The rhodopsin-bathorhodopsin forward and backward quantum yields sum to larger than one. These results are consistent with the previous suggestion that the excited singlet potential of rhodopsin has a single minimum along the 11-12 torsional coordinate. The values of the quantum yields are important for evaluating dynamic models of the rhodopsin-bathorhodopsin transition. We conclude that equilibration in the common excited state afer excitation of rhodopsin, as previously suggested, does not occur. Models involving molecular excitation trajectories conserving torsional momenta and excited state to ground state surface crossings better fit the data, and a semiquantitative analysis is presented. Probabilities of surface crossings are calculated.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aton B., Callender R. H., Honig B. Photochemical cis-trans isomerisation of bovine rhodopsin at liquid helium temperatures. Nature. 1978 Jun 29;273(5665):784–786. doi: 10.1038/273784a0. [DOI] [PubMed] [Google Scholar]
- Aton B., Doukas A. G., Narva D., Callender R. H., Dinur U., Honig B. Resonance Raman studies of the primary photochemical event in visual pigments. Biophys J. 1980 Jan;29(1):79–94. doi: 10.1016/S0006-3495(80)85119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. Energy uptake in the first step of visual excitation. Nature. 1979 Nov 29;282(5738):531–533. doi: 10.1038/282531a0. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G. The use of Ammonyx LO in the purification of rhodopsin and rod outer segments. Vision Res. 1971 Sep;11(9):1007–1009. doi: 10.1016/0042-6989(71)90220-3. [DOI] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R., Kropf A. THE ACTION OF LIGHT ON RHODOPSIN. Proc Natl Acad Sci U S A. 1958 Feb;44(2):130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G., Honig B., Ottolenghi M. Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts. Nature. 1977 Dec 8;270(5637):540–542. doi: 10.1038/270540a0. [DOI] [PubMed] [Google Scholar]
- Lewis A. The molecular mechanism of excitation in visual transduction and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):549–553. doi: 10.1073/pnas.75.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino M., Hamanaka T., Orii Y., Kito Y. Fractionation of rhodopsin and other components in the rod outer segment membrane by ammonium sulfate salting-out. Biochim Biophys Acta. 1977 Dec 20;495(2):299–311. doi: 10.1016/0005-2795(77)90386-5. [DOI] [PubMed] [Google Scholar]
- Oseroff A. R., Callender R. H. Resonance Raman spectroscopy of rhodopsin in retinal disk membranes. Biochemistry. 1974 Sep 24;13(20):4243–4248. doi: 10.1021/bi00717a027. [DOI] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichida Y., Tokunaga F., Yoshizawa T. Circular dichroism of squid rhodopsin and its intermediates. Biochim Biophys Acta. 1978 Dec 7;504(3):413–430. doi: 10.1016/0005-2728(78)90064-6. [DOI] [PubMed] [Google Scholar]
- Strackee L. Dichroism in the retina at -196 degrees C. Vision Res. 1970 Oct;10(10):925–938. doi: 10.1016/0042-6989(70)90070-2. [DOI] [PubMed] [Google Scholar]
- Strackee L. Photodichroism of rhodopsin solutions at -196 degrees C. Photochem Photobiol. 1972 Mar;15(3):253–268. doi: 10.1111/j.1751-1097.1972.tb07330.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Uji K., Kito Y. Studies on cephalopod rhodopsin: photoisomerization of the chromophore. Biochim Biophys Acta. 1976 Apr 23;428(2):321–338. doi: 10.1016/0304-4165(76)90040-4. [DOI] [PubMed] [Google Scholar]
- Warshel A. Charge stabilization mechanism in the visual and purple membrane pigments. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2558–2562. doi: 10.1073/pnas.75.6.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. TRANSFORMATIONS OF SQUID RHODOPSIN AT LOW TEMPERATURES. Nature. 1964 Jan 25;201:340–345. doi: 10.1038/201340a0. [DOI] [PubMed] [Google Scholar]
- van der Meer K., Mulder J. J., Lugtenburg J. A new facet in rhodopsin photochemistry. Photochem Photobiol. 1976 Oct;24(4):363–367. doi: 10.1111/j.1751-1097.1976.tb06837.x. [DOI] [PubMed] [Google Scholar]


