Abstract
In the present study we have exploited isogenic erg mutants of Saccharomyces cerevisiae to examine the contribution of an altered lipid environment on drug susceptibilities of yeast cells. It is observed that erg mutants, which possess high levels of membrane fluidity, were hypersensitive to the drugs tested, i.e., cycloheximide (CYH), o-phenanthroline, sulfomethuron methyl, 4-nitroquinoline oxide, and methotrexate. Most of the erg mutants except mutant erg4 were, however, resistant to fluconazole (FLC). By using the fluorophore rhodamine-6G and radiolabeled FLC to monitor the passive diffusion, it was observed that erg mutant cells elicited enhanced diffusion. The addition of a membrane fluidizer, benzyl alcohol (BA), to S. cerevisiae wild-type cells led to enhanced membrane fluidity. However, a 10 to 12% increase in BA-induced membrane fluidity did not alter the drug susceptibilities of the S. cerevisiae wild-type cells. The enhanced diffusion observed in erg mutants did not seem to be solely responsible for the observed hypersensitivity of erg mutants. In order to ascertain the functioning of drug extrusion pumps encoding the genes CDR1 (ATP-binding cassette family) and CaMDR1 (MFS family) of Candida albicans in a different lipid environment, they were independently expressed in an S. cerevisiae erg mutant background. While the fold change in drug resistance mediated by CaMDR1 remained the same or increased in erg mutants, susceptibility to FLC and CYH mediated by CDR1 was increased (decrease in fold resistance). Our results demonstrate that between the two drug extrusion pumps, Cdr1p appeared to be more adversely affected by the fluctuations in the membrane lipid environment (particularly to ergosterol). By using 6-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino-hexanoyl] sphingosyl phosphocholine (a fluorescent analogue of sphingomyelin), a close interaction between membrane ergosterol and sphingomyelin which appears to be disrupted in erg mutants is demonstrated. Taken together it appears that multidrug resistance in yeast is closely linked to the status of membrane lipids, wherein the overall drug susceptibility phenotype of a cell appears to be an interplay among drug diffusion, extrusion pumps, and the membrane lipid environment.
The incidence of the acquisition of resistance to azoles by Candida albicans cells has increased considerably in recent years, which has posed serious problems in successful chemotherapy of infections caused by such cells. Although the molecular basis of azole resistance in C. albicans is not very clear, accumulated evidence suggests that multidrug resistance (MDR) is a multifactorial phenomenon, with some of the most common mechanisms being the failure of drug accumulation mediated by drug extrusion pumps such as CDR1, CDR2 (ATP-binding cassette [ABC] family), and CaMDR1 (MFS family), alterations in Erg11p, and upregulation of ERG11. A combination of different resistance mechanisms has been reported to be responsible for fluconazole (FLC) resistance in clinical isolates of C. albicans (31, 41, 42, 44). Recent evidence also suggests that the generation of a resistant strain from a highly susceptible strain is the result of multiple mechanisms, each of which probably contributes partially to the resistant phenotype (23, 52). Of note, other mechanisms like the role of Δ5,6-desaturase (ERG3), Δ22-desaturase (ERG5), chromosomal alterations, modification of drugs, and membrane lipid composition which have been found to affect azole susceptibilities also merit consideration (21, 22, 27, 35, 37, 39).
On the basis of the results of several studies, a close interaction between membrane lipids and drug extrusion pump proteins has been realized (6, 7, 11, 45). On the one hand, it has been observed that the MDR ABC protein of mammalian cells (P glycoprotein [P-gp]) and MDR proteins of yeasts (Pdr5p and Yor1p in Saccharomyces cerevisiae and Cdr1p and Cdr2p in C. albicans) can translocate phospholipids between the two monolayers of the plasma membrane, while on the other hand P-gp has been shown to participate in sterol homeostasis in mammalian cells (5, 34). Additionally, these drug extrusion pumps are found to be particularly sensitive to the nature and the physical state of the surrounding lipids (11, 19, 47).
That lipid could also play an important role in azole susceptibilities is becoming apparent from a host of recent studies. It has been shown that some of the azole-resistant C. albicans isolates exhibit altered membrane phospholipid and sterol compositions (15, 23, 30). Such lipid changes are observed both in clinical and in in vitro-adapted azole-resistant isolates of C. albicans (16, 17, 23, 30). We as well as others have observed that Cdr1p and Pdr5p are sensitive to fluctuations in the lipid environment, where functions mediated by these drug extrusion pumps are selectively affected (19, 47). Taken together, it appears that the associated changes in membrane lipid composition (phospholipid and ergosterol), its order (fluidity), and asymmetry could be important determinants in the drug susceptibilities of yeast cells.
In order to study the contribution of membrane lipids in drug susceptibilities, in the present study we have exploited isogenic erg mutants of S. cerevisiae that, owing to blocks in different steps of ergosterol biosynthesis, possess increased levels of fluid membranes. We have also expressed two drug extrusion pumps, i.e., Cdr1p and CaMdr1p, in erg mutants to compare their functioning in an altered lipid environment (13, 43). We observed that erg mutants which possess higher levels of membrane fluidity were hypersensitive to the drugs tested. erg mutant cells elicit enhanced diffusion of drugs; however, that does not seem to solely affect drug susceptibilities. Furthermore, our results suggest that between the two pump proteins, Cdr1p, when it is expressed in erg mutants of S. cerevisiae, interacts more intimately with membrane lipids, particularly with ergosterol and sphingolipid, and thus is more severely affected by lipid perturbations.
MATERIALS AND METHODS
Materials.
Medium chemicals were obtained from Difco (Detroit, Mich.) and HiMedia (Mumbai, India). Labeled 6-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino-hexanoyl] sphingosyl phosphocholine (NBD-sphingomyelin [NBD-SM]) was purchased from Molecular Probes (Eugene, Oreg.), lipid N-rhodamine-dioleoyl phosphatidylethanolamine (N-Rh-DOPE) was purchased from Avanti Polar Lipids Inc. (Albaster, Ala), and l-dipalmitoyl-phosphatidylcholine (l-DPPC) was obtained from Sigma Chemical Co. (St. Louis, Mo.). The drugs cycloheximide (CYH), o-phenanthroline (PHE), 4-nitroquinoline oxide (4-NQO), and methotrexate (MTX) were from Sigma Chemical Co. FLC and sulfomethuron methyl (SMM) were provided as gifts by Pfizer (Sandwich, United Kingdom) and Dupont (Wilmington, Del.), respectively.
Strains used in this study.
Wild-type (WT) S. cerevisiae strain ABC287 (Matα ura3-52 leu2Δ1 lys2-801 his3Δ200 pep4Δ::HIS3 prb1Δ1.6R can1) and isogenic erg mutants designated erg2 (strain ABC271; Matα ura3-52 leu2Δ1 lys2-801 his3Δ200 pep4Δ::HIS3 prb1Δ1.6R can1 erg2Δ::LEU2), erg3 (strain ABC261; Matα ura3-52 leu2Δ1 lys2-801 his3Δ200 pep4Δ::HIS3 prb1Δ1.6R can1 erg3Δ::LEU2), erg4 (strain ABC283; Matα ura3-52 leu2Δ1 lys2-801 his3Δ200 pep4Δ::HIS3 prb1Δ1.6R can1 erg4Δ::LEU2), and erg6 (strain ABC265; Matα ura3-52 leu2Δ1 lys2-801 his3Δ200 pep4Δ::HIS3 prb1Δ1.6R can1 erg6Δ::LEU2) were used in this study and were kind gifts from Anand Bachhawat (IMTech, Chandigarh, India) (19). All the S. cerevisiae strains were transformed with plasmid pNC39 (carrying CaMDR1) and plasmid pS12 (carrying CDR1), which were obtained from a C. albicans genomic library, as described earlier (13, 43). Of note, both genes had a common vector background of pYEUra3. All erg mutants and WT strains of S. cerevisiae were maintained at 30°C in yeast nutrient broth (YNB) medium containing 2% glucose and the respective auxotrophic supplements (47).
Ergosterol extraction and estimation.
Sterols were extracted by the alcoholic KOH method as described by Arthington-Skaggs et al. (2, 3), in which cells grown overnight were harvested, washed in sterile water, and boiled for 1 h in 25% alcoholic KOH. The boiled cells were cooled and extracted with petroleum ether. In order to compare the efficiency of sterol extraction, yeast cells were also mechanically broken in an MSK homogenizer (Braun, Kronberg, Germany) prior to the sterol extraction. The homogenate was collected, and the sterols were extracted as described previously (2). An aliquot of sterol extract obtained by each protocol was diluted fivefold in petroleum ether and scanned spectrophotometrically between 200 and 320 nm (300 BIO UV-VIS spectrophotometer; Varian, Victoria, Australia). Both ergosterol and 24(28)-dehydroergosterol (DHE) absorb at 281.5 nm, whereas only DHE absorbs at 230 nm. The ergosterol content was calculated as a percentage of the wet weight of the cell by the following equations (2): percent ergosterol + percent DHE = [(A281.5/290) × F]/pellet weight, percent DHE = [(A230/518) × F]/pellet weight, and percent ergosterol = [percent ergosterol + percent DHE] − percent DHE, where F is the factor for dilution in petroleum ether, and 290 and 518 are the E values (in percent per centimeter) determined for crystalline ergosterol and DHE, respectively (2).
Supplementation of yeast cells and erg mutants with ergosterol.
For ergosterol supplementation experiments, cells were grown overnight in the presence of 10 μg of ergosterol ml−1, which had been dissolved in petroleum ether and added directly into the medium during inoculation (1). Sterols from supplemented cells were then extracted and analyzed by recording the wavelength scan between 200 and 320 nm as described above.
Fluorescence polarization studies.
The steady-state fluorescence polarization measurements on yeast cells were carried out essentially as described earlier (19). Measurements were carried out on whole cells by using a fluorescent probe, 1,6-diphenyl-1,3,5-hexatriene (DPH). Fluorescence polarization was measured at excitation and emission wavelengths of 360 and 450 nm, respectively. The measured fluorescence intensities were corrected for background fluorescence and the light scattering from the unlabeled sample (19).
Drug uptake assay.
Passive diffusion of rhodamine-6G (R6G) was determined essentially by a protocol described previously (23, 51). Approximately 107 cells from an overnight culture were inoculated in 250 ml of YPD and grown for 5 to 6 h at 30°C. The cells were pelleted and washed three times with phosphate-buffered saline buffer without glucose (buffer A). The cells were subsequently resuspended as a 2% cell suspension in deenergization buffer (5 mM dinitrophenol and 5 mM 2-deoxy-d-glucose in buffer A) and incubated for 2 h at 30°C. The cells were then washed; resuspended in buffer A, to which R6G was added to a final concentration of 10 μM; and incubated. An aliquot of 1 ml was taken at various times and centrifuged at 9,000 × g for 2 min. The absorbance of the supernatant was measured at 527 nm (23). The passive diffusion of FLC was checked by using radiolabeled [3H]FLC (24, 47). The deenergized cells were incubated with 100 nM [3H]FLC (0.7 TBq mmol−1); and aliquots were removed at various times, rapidly filtered, and washed three times with ice-cold buffer A containing an equimolar concentration of cold drug on a Millipore manifold filtration assembly with 0.45-μm-pore-size cellulose nitrate filter disks (Millipore, Bedford, Mass.). The radioactivity that accumulated in filtered cells and that adhered to filter disks was measured in a liquid scintillation counter with a scintillation liquid (Tri-Carb 2900TR; Packard). The radioactivity that adhered to the filter disk, which was not significant, was subtracted from the experimental values.
Drug susceptibility testing with S. cerevisiae strains.
The susceptibilities of the yeast isolates (grown in YNB medium containing 2% glucose and the respective auxotrophic supplements) to FLC, CYH, PHE, SMM, 4-NQO, and MTX were determined by three different methods. The following stock solutions of the various drugs were made (the solvent used is given in parentheses): FLC, 1 mg ml−1 (water); CYH, 0.1 mg ml−1 (water); PHE, 2 mg ml−1 (ethanol); 4-NQO, 0.01 mg ml−1 (dimethyl sulfoxide); SMM, 1 mg ml−1 (methanol); and MTX, 5 mg ml−1 (10 mM Tris-Cl). The resistance to MTX was checked in the presence of 200 μg of sulfanilamide ml−1 to deplete the intracellular dihydrofolate reductase pool, as previously described by Fling et al. (12). The solvents used to solubilize the different drugs were also tested, and there was no inhibition of growth due to the solvents used.
(i) Filter disk assay.
Yeast cells (105 cells ml−1) were mixed with molten agar (approximately 40°C) and poured in a petri plate. After agar solidification, the filter disks were placed and the drugs, in a volume of 5 to 10 μl, were spotted onto the disks at the indicated amounts: FLC, 100 μg; CYH, 0.5 μg; PHE, 20 μg; 4-NQO, 2 μg; SMM, 5 μg; and MTX, 50 μg. The plates were incubated at 30°C, and zones of inhibition were scored after 2 to 3 days (29, 38, 47).
(ii) Microtiter assay.
Cells were grown for 48 h at 30°C to obtain single colonies, which were resuspended in a 0.9% normal saline solution to give an optical density at 600 nm (OD600) of 0.1. The cells were then diluted 100-fold in YNB medium containing 2% glucose and the respective auxotrophic supplements. The diluted cell suspensions were added to the wells of round-bottomed 96-well microtiter plates (100 μl/well) containing equal volumes of medium (100 μl/well) and different concentrations of drugs (23, 50). A drug-free control was also included. The plates were incubated at 30°C for 48 h. The MIC test end point was evaluated both by eye and by reading the OD620 in a microplate reader and is defined as the lowest drug concentration that gave >80% inhibition of growth compared with the growth of the drug-free controls (the MIC at which 80% of isolates are inhibited [MIC80]).
(iii) Spot assay.
The yeast cells were grown overnight on YNB medium containing 2% glucose and the respective auxotrophic supplements. The cells were then suspended in normal saline to an OD600 of 0.1 (A600). Five microliters of fivefold serial dilutions of each yeast culture was spotted onto YNB plates in the absence (control) and presence of the following drugs: 4-NQO (0.02 μg ml−1), CYH (0.01 μg ml−1), PHE (2 μg ml−1), SMM (1 μg ml−1), FLC (8 μg ml−1), and MTX (1 μg ml−1). Growth differences were recorded following incubation of the plates for 48 h at 30°C. Growth was not affected by the presence of the solvents used for the drugs (8, 19, 53).
Labeling of cells with NBD-SM. (i) Preparation of donor lipid vesicles.
Donor vesicles containing fluorescent phospholipids were prepared by mixing the desired proportions of the various phospholipids as described by us and others (20, 48). The typical proportion used was 40 mol% NBD-SM, 2 mol% N-Rh-DOPE, and 58 mol% l-DPPC. After all of these phospholipids were mixed, chloroform was evaporated under a stream of N2 to make a thin film and kept overnight under vacuum desiccation to remove moisture and traces of solvent. Desiccated phospholipid film was then hydrated by adding 50 mM Tris buffer (pH 7.4; buffer A) with continuous vortexing. Hydrated membrane film was then sonicated for 30 min under an N2 atmosphere with a water bath sonicator to produce donor vesicles.
(ii) Labeling of C. albicans cells and erg mutants with NBD-SM.
Labeling of cells with fluorescent lipids was carried out essentially as described earlier (20, 48). Briefly, cells grown to the mid-log phase were harvested by centrifugation at 3,000 rpm for 5 min, washed two times with buffer B, and resuspended (5 × 109 cells ml−1) in the same buffer. Donor vesicles (final concentration, 40 μM) were then added to 900 μl of the cell suspension, and every 5 min the fluorescence of the cell suspension was measured spectrofluorometrically (excitation wavelength, 475 nm; emission wavelength, 525 nm; eclipse spectrofluorometer; Varian) to check the labeling of the cells by NBD-SM. After 90 min, when labeling of the cells attained saturation, the labeled cells were washed twice with ice-cold buffer B and resuspended in 900 μl of the same buffer (5 × 109 cells ml−1). Then, fatty acid-free bovine serum albumin (BSA; final concentration, 2% [wt/vol]) was added to the cell suspension to allow the back exchange of NBD-SM from the outer monolayer of the plasma membrane to BSA. At different time intervals, aliquots of the cell suspension were taken out and centrifuged, and the fluorescence in both the supernatant and the pellet was measured. Fluorescence intensities were corrected for background fluorescence in both the supernatant and the pellet. The BSA-extracted NBD-SM fraction was calculated as follows: percent NBD-SM extracted = (fluorescence of supernatant/total fluorescence from supernatant and pellet) × 100.
RESULTS
S. cerevisiae erg mutant strains lack ergosterol.
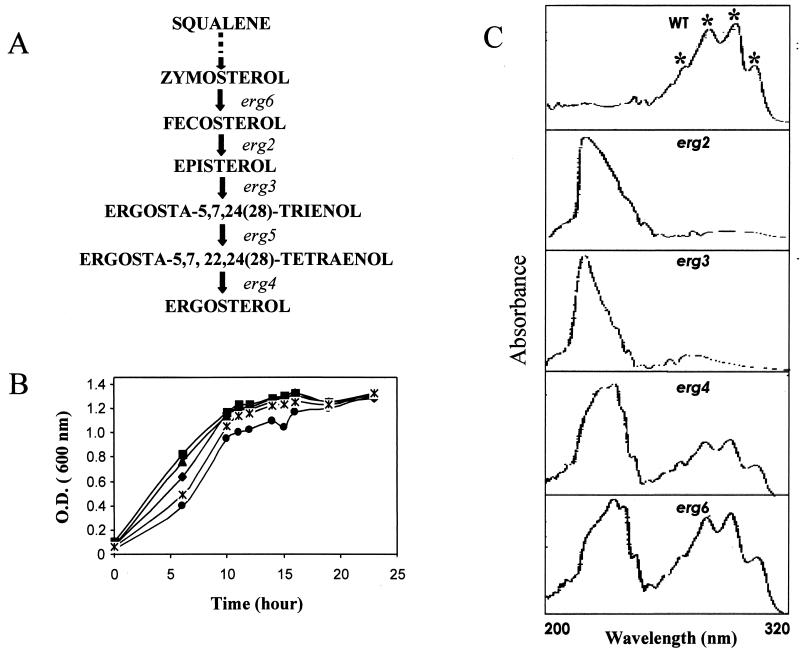
Mutants with mutations in the ergosterol biosynthetic pathway do not produce ergosterol and utilize sterol intermediates to compensate for the loss of ergosterol (19, 28, 47) (Fig. 1A). To confirm the absence of any detectable levels of ergosterol in erg mutant strains, we compared the absorption spectra of sterols obtained from an S. cerevisiae WT strain and its isogenic erg mutants (1-3, 40). The method of Arthington-Skaggs et al. (2, 3) takes advantage of the unique four-peak spectral absorption pattern produced by extracted sterols between 240 and 320 nm; this pattern is indicative of the ergosterol and DHE (a late sterol pathway intermediate) contents. Both ergosterol and DHE absorb at 281.5 nm, whereas only DHE shows an intense spectral absorption band at 230 nm. Therefore, the amount of ergosterol can be determined by calculating the total ergosterol plus DHE content (by determination of the amount of absorption at 281.5 nm) and then subtracting that amount from the total amount of absorption (at 230 nm) due to DHE only (2, 3). As evident from Fig. 1C, WT cells gave four characteristics peaks for ergosterol (no absorption peak at 230 nm) which were absent for both erg2 and erg3 mutants. Mutant erg6 and erg4 cells, however, gave four characteristic peaks as well as a strong absorption peak for DHE at 230 nm. Quantitation of ergosterol from the absorption spectra (see Materials and Methods) confirmed that the WT strain had an ergosterol content of 1.3% ± 0.07% (expressed as a percentage of the wet weight of the cells), whereas none of four erg mutants contained ergosterol (data not shown).
FIG. 1.
(A) Schematic representation of the late stages of the ergosterol biosynthetic pathway. erg6, erg2, erg3, erg5, and erg4 encode S-adenosyl methionine methyltransferase, C-8 sterol isomerase, sterol C-5 desaturase, C-22 sterol desaturase, and sterol-24(28) reductase, respectively. (B) Growth curves of WT (♦), erg2 (▪), erg3 (▴), erg4 (•), and erg6 (∗) strains. The specific growth rates are 1.76 h for WT, 2.01 h for erg2, 1.78 h for erg3, 1.52 h for erg4, and 1.71 h for erg6. (C) UV absorption spectra of sterols extracted from the S. cerevisiae WT strain and its erg mutants. Their absorption spectra were recorded as described in Materials and Methods.
erg mutants of S. cerevisiae are susceptible to drugs.
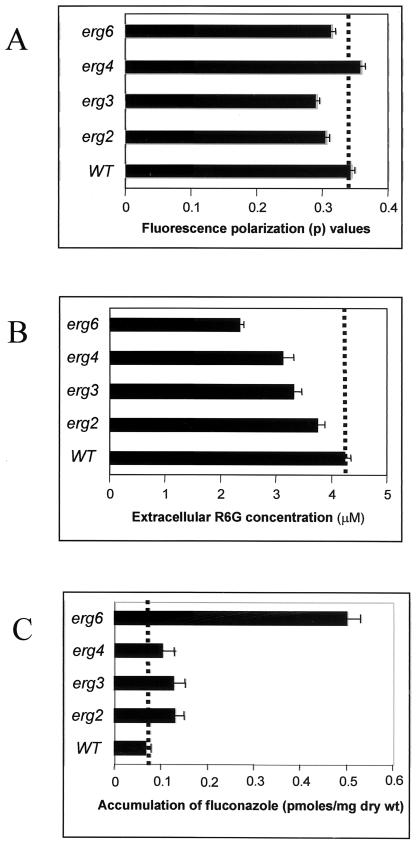
In order to ascertain the physical state of the membrane, we used steady-state fluorescence polarization to examine the membrane order (fluidity) of erg2, erg3, erg4, and erg6 mutants and the WT strain. A specific plasma membrane fluorescent probe, DPH, was used for such measurements, as described earlier (1, 19). All the erg mutant strains showed decreases in fluorescence polarization, thus implying decreased membrane order or enhanced fluidity of the membranes of these mutants compared to that for the WT strain (Fig. 2A). However, we did not observe any significant change in fluidity of the membrane of the erg4 strain, which is probably because it mediates the final step of ergosterol biosynthetic pathway in yeast. In the plasma membrane of the erg4 mutant, ergosterol is completely replaced with ergosta-5,7,22,24(28)-tetraen-3β-ol, which is structurally highly related to ergosterol and thus is able to completely compensate for it in the membrane (53).
FIG. 2.
(A) Steady-state fluorescence polarization measurements (p value) in an S. cerevisiae WT strain and its erg mutants. Measurements were carried out with intact cells by using DPH as the fluorescent probe at excitation and emission wavelengths of 360 and 450 nm, respectively, as described in Materials and Methods. The values are means ± standard deviations (indicated by the bars) of three independent experiments. (B) Extracellular R6G concentration measured in S. cerevisiae WT and its erg mutants at 60-min interval. Deenergized yeast cells were incubated with R6G for 1 h, after which the cells were harvested and the extracellular concentration of R6G in the supernatant was determined spectrophotometrically by measuring the absorbance at 527 nm. The values are means ± standard deviations (indicated by the bars) of three independent experiments. (C) Accumulation of [3H]FLC in deenergized S. cerevisiae WT and its erg mutants at 60-min intervals. The values are means ± standard deviations (indicated by the bars) of three independent experiments.
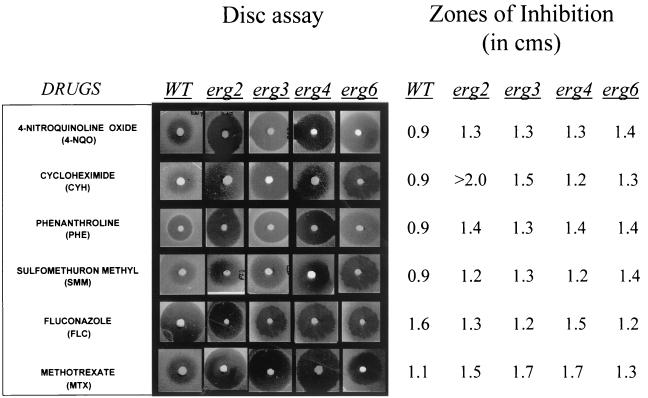
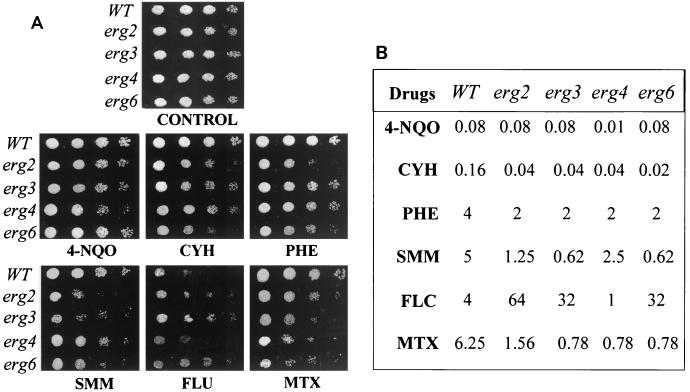
Whether the enhanced membrane fluidity of erg mutants in any way affects the drug susceptibilities of the mutant cells was checked in the following experiments. Since there is no recommended protocol for testing of the drug susceptibilities of S. cerevisiae cells, we simultaneously used three independent methods (filter disk, spot, and microtiter plate assays) to examine the drug susceptibilities of the erg mutants (8, 36, 38). As evident from the results of the filter disk assay (Fig. 3), all erg mutants were highly sensitive to most of the drugs tested, which was evident from the sizes of the zones of inhibition. The drug sensitivity was further confirmed by the microtiter assay, in which MIC80s of the drugs for the cells of the erg mutant strains were lower than those for the WT cells (Fig. 4B). The drug sensitivities tested by spot assays also revealed that the mutant strains were generally sensitive to the drugs tested (Fig. 4A). Thus, the results from three independent drug susceptibility tests led us to confirm the results of the resistance profiling of the erg mutants. Notably, all erg mutant strains except strain erg4 were highly resistant to FLC; strain erg4 was even more susceptible than the WT (MIC80s, 1 μg ml−1 for erg4 versus 4 μg ml−1 for the WT). By the disk assay, however, no major differences in the FLC susceptibilities of erg4 and other mutant cells were evident. This points out that sometimes the MIC and the result of the filter disk assay may not match (10). Since there was no significant difference between the growth of the erg mutants and the WT cells (Fig. 1B), differences in growth could not have contributed to the observed variations in drug susceptibilities. The observed resistance of erg mutants of S. cerevisiae to FLC needs special mention. While our observation is limited to the erg mutants of S. cerevisiae studied, evidence suggests that mutations in ERG genes could also affect azole sensitivity in C. albicans cells. The accumulation of ergosta-7,22-dienol-3β-ol in an azole-resistant clinical isolate of C. albicans has been attributed to the absence of ERG3 (Δ5,6-desaturase) (21, 33, 35, 44). Additionally, the susceptibility patterns of erg mutants of Candida could be different. In the present study we observed that the erg6 mutant of S. cerevisiae is resistant to FLC, while Jensen-Pergakes et al. (18) showed that a C. albicans strain with the same mutation has nearly identical susceptibilities to FLC and other azoles.
FIG. 3.
Drug resistance profiles of S. cerevisiae WT and erg mutants determined by filter disk assay as described in Materials and Methods. The numbers on the right represent zones of inhibition measured from the zones shown on the left.
FIG. 4.
Drug resistance profiles of S. cerevisiae WT and erg mutants determined by the spot assay (A) and the microtiter assay (B). (A) For the spot assay, the yeast cells were grown overnight on YNB plates at 30°C. The cells were then suspended in normal saline to an OD600 of 0.01 (A600). Five microliters of fivefold serial dilutions of each yeast culture was spotted onto YNB plates in the absence (control) and presence of the following drugs: 4-NQO (0.02 μg ml−1), CYH (0.01 μg ml−1), PHE (2 μg ml−1), SMM (1 μg ml−1), FLC (8 μg ml−1), and MTX (1 μg ml−1). Growth differences were recorded following incubation of the plates for 48 h at 30°C. Growth was not affected by the presence of the solvents used for the drugs (B) The microtiter assay (MIC80) was done as described in Materials and Methods.
Membrane lipid phase affects passive diffusion of drugs.
In order to test the possible influence of enhanced membrane fluidity on the rate of drug import via passive diffusion, we first excluded the contributions of efflux pumps, which could interfere with diffusion measurements. This was achieved by energy depletion of cells, wherein S. cerevisiae cells were aerated for 1 h in the presence of 5 mM 2-deoxy-d-glucose and 5 mM 2,4-dinitrophenol, which was sufficient to deplete most of the intracellular ATP (data not shown) (51). The yeast cells were then incubated in the presence of the fluorescent substrate R6G as described in Materials and Methods. The drug diffusion in energy-depleted cells was determined by measuring absorbance changes in the R6G contents in the supernatant withdrawn at different times. It is evident from Fig. 2B that after 1 h of incubation in the presence of R6G, the supernatants of all the mutant strains showed low extracellular R6G concentrations, implying increased entry (passive diffusion) of the fluorophore into the cells. The enhanced accumulation of [3H]FLC (Fig. 2C) in energy-depleted cells (diffusion) also confirmed that erg mutants elicit high levels of diffusion. Thus, the drug sensitivities of the erg mutants could be due at least in part to an enhanced passive diffusion of the drugs, which is influenced by the membrane lipid phase.
In vitro-induced changes in membrane fluidity do not affect drug resistance.
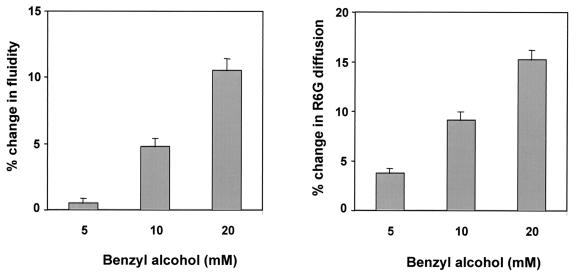
In order to further explore if the physical state of membrane lipids can indeed influence the drug resistance of yeast cells, we induced changes in the membrane fluidity by a known membrane fluidizer, benzyl alcohol (BA) (46). The addition of 5 to 20 mM BA led to a gradual increase in membrane fluidity which was maximally enhanced to 10 to 12% in cells exposed to 20 mM BA (Fig. 5). The enhanced fluidity also led to an increase in the diffusion of R6G by 15%. However, the BA-induced changes in fluidity and passive diffusion did not alter the sensitivities to the drugs tested (data not shown). It is thus probable that enhanced membrane fluidity and the diffusion therefrom may not be the only cause of the hypersensitivity observed for the erg mutants.
FIG. 5.
Measurement of membrane fluidity and passive diffusion of S. cerevisiae WT cells induced by BA. Fluidity was determined by measuring the steady-state fluorescence polarization with DPH as the fluorescent probe as described in the legend for Fig. 2A. Diffusion was determined by measuring the extracellular R6G concentration as described in the legend for Fig. 2B. The values are means ± standard deviations (indicated by the bars) of three independent experiments.
Membrane environment selectively affects drug extrusion pumps of Candida when expressed in S. cerevisiae.
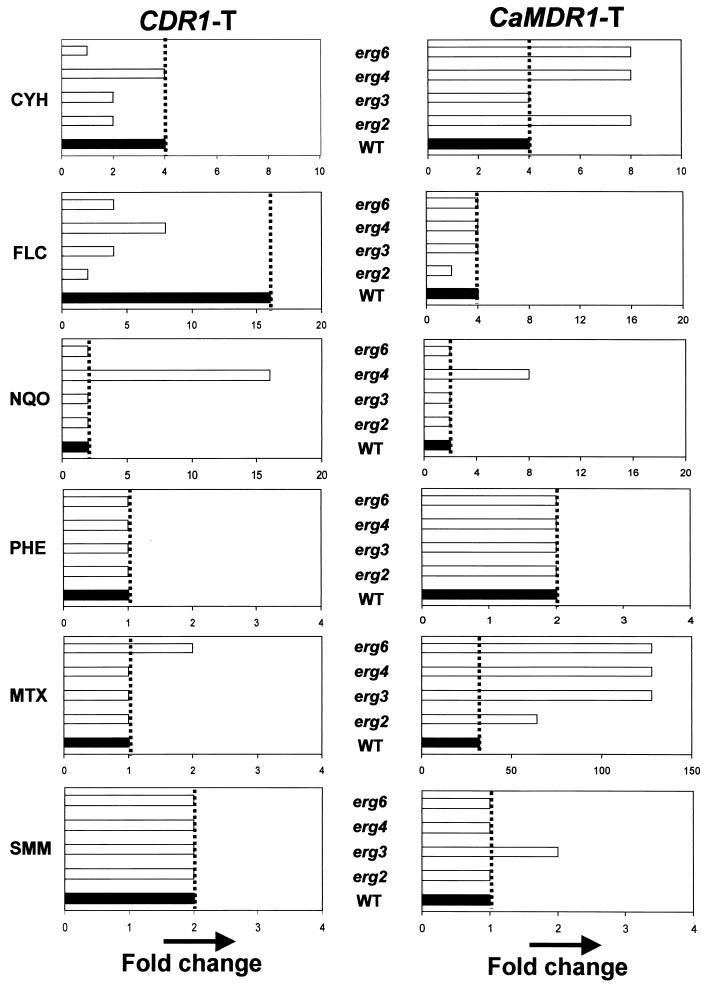
In view of the intricate relationship between the membrane lipid phase and the membrane proteins, we examined the functioning of two MDR pumps of C. albicans, Cdr1p and CaMdr1p, in an altered membrane environment. Both the WT and erg mutants of S. cerevisiae were transformed with the CDR1 and CaMDR1 genes cloned on an identical vector, as described in Materials and Methods. The levels of expression of CDR1 and CaMDR1 were unaffected in erg mutants compared to the levels of expression in the WT strain (data not shown). The drug resistance conferred by the levels of expression of these proteins in an altered membrane environment was examined by a microtiter plate method (Fig. 6). Since erg mutant strains displayed different levels of hypersensitivity to the drugs compared to the sensitivity of the WT strain (Fig. 3 and 4), we calculated the fold change (Fig. 6) in resistance of the yeast transformants from that of their respective nontransformant strains. The Cdr1p-mediated drug resistance appeared to be adversely affected by the lipid environment. For example, the ability of Cdr1p to mediate resistance to CYH (which is a preferred substrate for this pump) was severely hampered (two- to fourfold decrease), especially in the erg2, erg3, and erg6 mutant backgrounds. The fold resistance to FLC by Cdr1p was also decreased in the erg mutant strains, while the levels of resistance to 4-NQO, MTX, SMM, and PHE remained unaffected (Fig. 6). Interestingly, CaMdr1p appeared to be insensitive to membrane alterations since the fold increase in resistance that it mediated remained largely unchanged or increased between the WT and its erg mutant strains (Fig. 6). The resistance to FLC mediated by Cdr1p needs special mention. It is evident from Fig. 3 and 4 that erg mutants are intrinsically resistant to FLC. The WT strain, which was sensitive to FLC, exhibited a 16-fold higher level of resistance when CDR1 was expressed in this background. On the other hand, expression of CaMDR1 in the WT yeast strain resulted in only a fourfold enhanced level of resistance. Interestingly, while the fold resistance to FLC is more or less maintained in the case of CaMDR1 expression, CDR1-transformed erg mutant cells could not sustain the level of resistance elicited in WT cells.
FIG. 6.
Graphical representations showing the fold increase in drug resistance of WT and erg mutant strains of S. cerevisiae upon transformation with Cdr1p- and CaMdr1p-encoding plasmids (see Materials and Methods). The fold increase in resistance to each drug was calculated as the difference in resistance between the nontransformant and transformant pair for each cell type. MTX is the substrate for CaMdr1p (24), and the fold change in resistance is much higher for CaMDR1-transformed erg mutant cells than for CDR1-transformed erg mutant cells. Note the change in the x axis for fold MTX resistance.
Cdr1p is more sensitive to lipid environment.
It became clear from the results presented above that, compared to CaMdr1p, which is an MFS transporter, ABC drug transporter Cdr1p is more sensitive to lipid perturbations. In order to explore such an interaction between membrane ergosterol and sphingomyelin, we preformed the experiment described below.
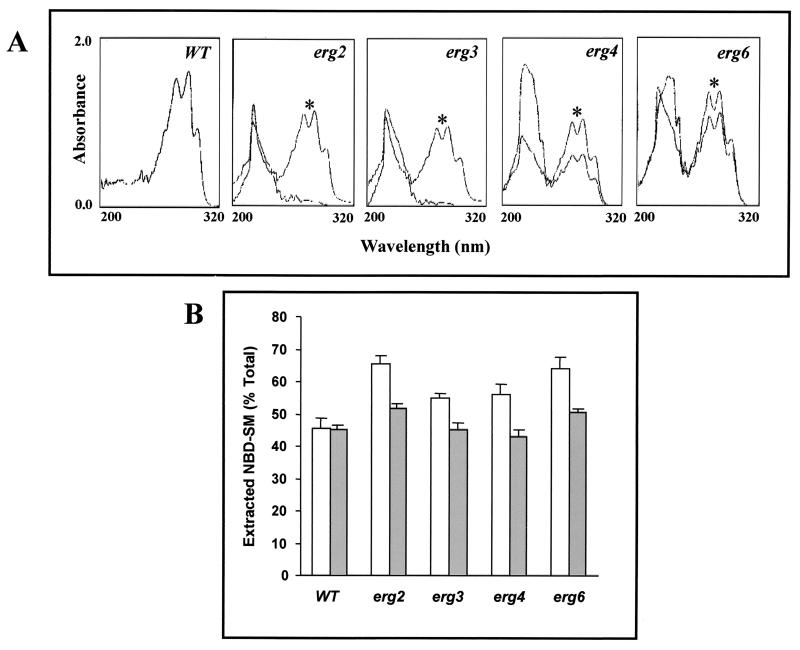
We labeled WT and erg mutant cells of S. cerevisiae with NBD-SM. The important advantage of this lipid analogue is that it can readily be inserted into biological membranes by spontaneous lipid transfer from exogenous carriers (20, 48). At 90 min postlabeling, when the level of NBD-SM incorporation was maximal, the cells were washed and the associated NBD-SM of the labeled cells was back extracted by using 2% fatty acid-free BSA. The labeling protocol and back exchange of labeled NBD-SM were described in Materials and Methods and in an earlier publication (48). Figure 7B depicts the exchangeability of NBD-SM from different labeled strains of S. cerevisiae. It is clear that the efficiency of extraction of NBD-SM by BSA from erg mutants is greater than that from the WT (Fig. 7B). It probably suggests that ergosterol depletion in erg mutant cells results in disruption of the interaction between ergosterol and sphingomyelin, which leads to the enhanced exchange of NBD-SM. In order to examine the lipid interaction more closely, the growth media of erg mutants was supplemented with ergosterol. That supplemented ergosterol is incorporated into the membrane was confirmed by spectral analysis. Figure 7A shows the appearance of characteristic peaks of ergosterol in supplemented cells. Interestingly, the exchange of NBD-SM was considerably reduced (restored to the level for WT cells) when erg mutant cells were grown in ergosterol-supplemented media (Fig. 7B). This shows that the ergosterol content affects the exchangeability of sphingomyelin and thus implies a close interaction between the two membrane lipid components.
FIG. 7.
(A) UV absorption spectra of sterols extracted from the S. cerevisiae WT strain and its erg mutants grown in the absence and in the presence (∗) of ergosterol. The cells were supplemented with 10 μg of ergosterol ml−1 and grown for 20 h at 30°C before lipid extraction. The supplemented cells were harvested and washed thoroughly before extraction of sterols as described in Materials and Methods. The UV spectra of WT cells were the same as those of the unsupplemented cells; hence, only one trace is shown in the first panel. (B) Postlabeling transbilayer exchange of NBD-SM in the S. cerevisiae WT and its erg mutants. Cells were grown in the absence (open bar) and presence (filled bar) of ergosterol-supplemented media as described in Materials and Methods. NBD-SM labeled cells were washed twice with buffer A and were then incubated with 2% BSA to back exchange the NBD-SM from the labeled cells, as described in Materials and Methods. The percentage of total extracted NBD-SM was calculated (according to the formula given in Materials and Methods). The graph presents data for the 90-min time point during which the maximum back-exchanged fluorescence in the supernatant was observed. The values are the means ± standard deviations (indicated by the bars) of three independent experiments.
DISCUSSION
In this study we have presented evidence highlighting the close interaction between membrane lipids and the drug sensitivities of yeast cells. For this we used erg2, erg3, erg4, and erg6 mutants of S. cerevisiae which are blocked in late ergosterol biosynthesis (Fig. 1A). Due to defective ergosterol biosynthesis and, as a result, the accumulation of various intermediates, these mutants possessed high levels of membrane fluidity. These mutants were in general sensitive to several of the drugs tested; however, they were resistant to FLC (the only azole tested). The hypersensitivities of the yeast erg mutants could be attributed to membrane permeability changes which may involve changes in passive diffusion across the membrane or in the active transport of these drugs. In support of the former, Van Den Hazel et al. (51) have shown that the lack of PDR16 and PDR17 (which encode homologues of Sec14p) S. cerevisiae cells results in altered phospholipid and sterol compositions and renders cells hypersensitive to many drugs due to their increased passive diffusion. In support of the latter possibility, the ABC transporter Pdr5p has been shown to function less efficiently in cells from which erg6 is deleted. A recent report by Emter et al. (9), however, suggests that ERG6 of S. cerevisiae increases the rate of passive drug diffusion without affecting Pdr5p-mediated drug export. In this study we used two substrates, i.e., the fluorophore R6G and radiolabeled FLC, and observed that erg mutants elicited higher levels of passive diffusion. This suggests that the enhanced diffusion of drugs could contribute to the hypersensitivities of the erg mutants. However, in another set of experiments, when we increased the membrane fluidity by using a membrane fluidizer, BA, we observed that a 10 to 12% increment in fluidity did not affect the susceptibilities of the S. cerevisae cells to the drugs tested. Taken together, it appears that the change in membrane fluidity and the increased diffusion therefrom alone may not be sufficient to result in the observed higher susceptibilities of the erg mutants. Since these mutants have altered sterol compositions, it is likely that sterol interactions with other membrane lipids and transporters could be more relevant to the higher drug susceptibilities observed.
We observed that when the MFS transporter CaMdr1p and the ABC transporter Cdr1p of C. albicans are expressed in erg mutants of S. cerevisiae, the latter appears to be more responsive to the fluctuations in the membrane lipid environment. We show that when Cdr1p is expressed in erg mutants it could not restore the level of resistance to CYH and FLC compared to the level of resistance obtained when it was expressed in WT cells. Interestingly, two independent observations suggest that drug extrusion pump sensitivity to the lipid environment was not a general phenomenon: first, the Cdr1p-mediated levels of resistance to drugs like NQO, SMM, PHE, and MTX remained unchanged when Cdr1p was expressed in erg mutants, while the sensitivities to CYH and FLC changed drastically; second, the CaMdr1p-mediated levels of resistance to similar drugs remained unaffected and were rather improved in some instances when CaMdr1p was expressed in an erg background. Taken together, our results suggest that the Cdr1p ABC drug extrusion pump is selectively more sensitive to fluctuations in sterol composition.
Human P-gp, a homologue of Cdr1p, is also shown to be sensitive to fluctuations in the membrane lipid environment, where it intimately interacts with membrane sphingomyelin and cholesterol (32). In this study, we also observed a close interaction between membrane sterol (ergosterol in this case) and sphingolipids. We were able to exchange larger amounts of NBD-SM from erg mutants which could be reversed if the mutants were supplemented with ergosterol. A very recent (49) report shows that regulation of sphingolipid metabolism is sterol dependent in S. cerevisiae. This again signifies the mutual importance of the two membrane lipids in yeast (4).
Interestingly, Cdr1p-mediated phospholipid translocase activity was found to be independent of ergosterol depletion (47). This shows how functional domains of a protein respond differently to fluctuations in the lipid environment. Such selective sensitivity of an efflux pump protein (Cdr1p) to fluctuations in the membrane lipid environment (loss of resistance to certain drugs) and selectivity within the efflux proteins (Cdr1p is more sensitive to lipid fluctuation than CaMdr1p) do point to very specific interactions between efflux pump proteins and membrane lipids.
Recently, Kontoyiannis (25) has provided genetic evidence that Pdr5p-mediated resistance to FLC is altered in the background of mutations that affect ergosterol homeostasis. It is suggested that the ABC protein functions in the maintenance of ergosterol homeostasis by extrusion or transport of sterol intermediates. In view of the observed close interaction between ergosterol and sphingomyelins in the yeast membrane, such homeostasis is expected to be perturbed in erg mutants and could affect the functioning of drug extrusion pump proteins (14). That mitochondria and oxidative phosphorylation are physiological partners of at least Erg3p is another aspect which merits attention (26). In view of the compelling evidence from various sources in which differences in the membrane ultrastructures and the sterol and phospholipid compositions between drug-resistant and -sensitive cell lines (for a review, see reference 11) and yeast cells (30) are observed, it is important to assess the roles of such changes in the overall scenario of multidrug resistance.
Acknowledgments
The work presented in this paper has been supported in parts by grants (to R.P.) from the Department of Biotechnology (DBT-BT/PRO798/HRD20/8/98), the Department of Science and Technology (SP/SO/D57/97), and the Council of Scientific and Industrial Research [60 (0028)/98-EMR-II] of India; the European Commission (QLK2-CT-2001-02377), Brussels, Belgium; and the Volkswagen Foundation (1/76 798), Hannover, Germany. A.K. acknowledges the fellowship award from the University Grants Commission, and K.M. acknowledges the Scientist Pool Scheme of the Council of Scientific and Industrial Research.
Kasturi Mukhopadhyay and Avmeet Kohli contributed equally to this work.
REFERENCES
- 1.Ansari, S., P. Gupta, S. K. Mahanty, and R. Prasad. 1993. The uptake of amino acids by erg mutants of Candida albicans. J. Med. Vet. Mycol. 31:377-386. [Google Scholar]
- 2.Arthington-Skaggs, B. A., H. Jradi, T. Desai, and C. J. Morrison. 1999. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 37:3332-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthington-Skaggs, B., W. Lee-Yang, M. A. Ciblak, J. P. Frade, M. E. Brandt, R. A. Hajjeh, L. H. Harrison, A. N. Sofair, and D. W. Warnock. 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 46:2477-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagnat, M., S. Keranen, A. Shevchenko, A. Shevchenko, and K. Simons. 2000. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA 97:3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debry, P., E. A. Nash, D. W. Nekalson, and J. E. Metherall. 1997. Role of multidrug resistance P-glycoproteins in cholesterol esterification. J. Biol. Chem. 272:1026-1031. [DOI] [PubMed] [Google Scholar]
- 6.Decottignies, A., A. M. Grant, J. W. Nichols, H. De Wet, D. B. McIntosh, and A. Goffeau. 1998. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem. 273:12612-12622. [DOI] [PubMed] [Google Scholar]
- 7.Dogra, S., S. Krishnamurthy, V. Gupta, B. L. Dixit, C. M. Gupta, D. Sanglard, and R. Prasad. 1999. Asymmetric distribution of phosphatidylethanolamine in C. albicans: possible mediation by CDR1, a multidrug transporter belonging to ATP binding cassette (ABC) superfamily. Yeast 15:111-121. [DOI] [PubMed] [Google Scholar]
- 8.Egner, R., F. E. Rosenthal, A. Kralli, D. Sanglard, and K. Kuchler. 1998. Genetic separation of FK506 susceptibility and drug transport in the yeast Pdr5 ATP-binding cassette multidrug resistance transporter. Mol. Biol. Cell 9:523-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emter, R., A. Hesse-Peck, and A. Kralli. 2002. ERG6 and PDR5 regulate small lipophilic drug accumulation in yeast cells via distinct mechanisms. FEBS Lett. 521:57-61. [DOI] [PubMed] [Google Scholar]
- 10.Espinell-Ingroff, A., F. Barchiesi, K. C. Hazen, J. V. Martinez-Suarez, and G. Scalise. 1998. Standardization of antifungal susceptibility testing and clinical relevance. Med. Mycol. 36:68-78. [PubMed] [Google Scholar]
- 11.Ferte, J. 2000. Analysis of the tangled relationships between P-glycoprotein-mediated multidrug resistance and the lipid phase of the cell membrane. Eur. J. Biochem. 267:277-294. [DOI] [PubMed] [Google Scholar]
- 12.Fling, M. E., J. Kopf, A. Tamarkin, J. A. Gorman, H. A. Smith, and Y. Koltin. 1991. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol. Gen. Genet. 227:318-329. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, V., A. K. Kohli, S. Krishnamurthy, N. Puri, S. A. Aalamgeer, S. L. Panwar, and R. Prasad. 1998. Identification of polymorphic mutant alleles of CaMDR1, a major facilitator of Candida albicans which confers multidrug resistance and its in vitro transcriptional activation. Curr. Genet. 34:192-199. [DOI] [PubMed] [Google Scholar]
- 14.Hallstrom, C. T., L. Lambert, S. Schorling, E. Balzi, A. Goffeau, and W. S. Moye-Rowley. 2001. Coordinate control of sphingolipid biosynthesis and multidrug resistance in Saccharomyces cerevisiae. J. Biol. Chem. 26:23674-23680. [DOI] [PubMed] [Google Scholar]
- 15.Hitchcock, C. A. 1993. Resistance of Candida albicans to azole antifungal agents. Biochem. Soc. Trans. 21:1039-1047. [DOI] [PubMed] [Google Scholar]
- 16.Hitchcock, C. A., K. Barrett-Bee, and N. J. Russel. 1986. The lipid composition of azole-sensitive and azole-resistant strains of Candida albicans. J. Gen. Microbiol. 132:2421-2431. [DOI] [PubMed] [Google Scholar]
- 17.Hitchcock, C. A., N. J. Russel, and K. J. Barrett-Bee. 1987. Sterols in Candida albicans mutants resistant to polyene or azole antifungals, and of a double mutant C. albicans. CRC Crit. Rev. Microbiol. 15:111-115. [DOI] [PubMed] [Google Scholar]
- 18.Jensen-Pergakes, K. L., M. A. Kennedy, N. D. Lees, R. Barbuch, C. Koegel, and M. Bard. 1998. Sequencing, disruption, and characterization of the Candida albicans sterol methyltransferase (ERG6) gene: drug susceptibility studies in erg6 mutants. Antimicrob. Agents Chemother. 42:1160-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur, R., and A. K. Bachhawat. 1999. The yeast multidrug resistance pump, Pdr5p, confers reduced drug resistance in erg mutants of Saccharomyces cerevisiae. Microbiology 145:809-818. [DOI] [PubMed] [Google Scholar]
- 20.Kean, L. S., A. M. Grant, C. Angeletti, Y. Mahe, K. Kuchler, R. S. Fuller, and J. W. Nichols. 1997. Plasma membrane translocation of fluorescent-labeled phosphatidylethanolamine is controlled by transcription regulators, PDR1 and PDR3. J. Cell Biol. 138:255-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly, S. L., D. C. Lamb, D. E. Kelly, J. Loffler, and H. Einsele. 1996. Resistance to fluconazole and amphotericin in Candida albicans from AIDS patients. Lancet 348:1523-1524. [DOI] [PubMed] [Google Scholar]
- 22.Kelly, S. L., D. C. Lamb, D. E. Kelly, N. J. Manning, J. Loeffler, H. Hebart, U. Schumacher, and H. Einsele. 1997. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol Δ5,6-desaturation. FEBS Lett. 400:80-82. [DOI] [PubMed] [Google Scholar]
- 23.Kohli, A., Smriti, K. Mukhopadhyay, A. Rattan, and R. Prasad. 2002. In vitro low-level resistance to azoles in Candida albicans is associated with changes in membrane lipid fluidity and asymmetry. Antimicrob. Agents Chemother. 46:1046-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohli, A., V. Gupta, S. Krishnamurthy, S. E. Hasnain, and R. Prasad. 2001. Specificity of drug transport mediated by CaMDR1: a major facilitator of Candida albicans. J. Biosci. 26:333-339. [DOI] [PubMed] [Google Scholar]
- 25.Kontoyiannis, D. P. 2000. Efflux mediated resistance to fluconazole could be modulated by sterol homeostasis in Saccharomyces cerevisiae. J. Antimicrob. Chemother. 46:199-203. [DOI] [PubMed] [Google Scholar]
- 26.Kontoyiannis, D. P. 2000. Modulation of fluconazole sensitivity by the interaction of mitochondria and Erg3p in Saccharomyces cerevisiae. J. Antimicrob. Chemother. 46:191-197. [DOI] [PubMed] [Google Scholar]
- 27.Lamb, D. C., S. Maspahy, D. E. Kelly, N. J. Manning, A. Geber, J. E. Bennett, and S. L. Kelly. 1999. Purification, reconstitution, and inhibition of cytochrome P-450 sterol Δ22-desaturase from the pathogenic fungus Candida glabrata. Antimicrob. Agents Chemother. 43:1725-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lees, N. D., B. Skaggs, D. R. Kirsch, and M. Bard. 1995. Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae—a review. Lipids 30:221-226. [DOI] [PubMed] [Google Scholar]
- 29.Leppert, G., R. McDevitt, S. C. Falco, F. I. M. Van Dyk, and J. Golin. 1990. Cloning by gene amplification of two loci conferring multiple drug resistance in Saccharomyces. Genetics 125:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loffler, J., H. Einsele, H. Hebart, U. Schumacher, C. Hrastnik, and G. Daum. 2000. Phospholipid and sterol analysis of plasma membranes of azole-resistant Candida albicans strains. FEMS Microbiol. Lett. 185:59-63. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Ribot, J. L., R. K. McAtee, S. Perea, W. R. Kirkpatrick, M. G. Rinaldi, and T. F. Patterson. 1999. Multiple resistant phenotypes of Candida albicans coexist during episodes of oropharyngeal candidiasis in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 43:1621-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luker, G. D., C. M. Pica, A. S. Kumar, D. F. Covey, and D. Piwnica-Worms. 2000. Effects of cholesterol and enantiomeric cholesterol on P-glycoprotein localization and function in low-density membrane domains. Biochemistry 39:7051-7661. [DOI] [PubMed] [Google Scholar]
- 33.Marichel, P., H. Vanden Bossche, F. C. Odds, G. Nobels, D. W. Warnock, V. Timmerman, C. Van Broeckhoven, S. Fay, and P. Mose-Larsen. 1997. Molecular-biological characterization of an azole-resistant Candida glabrata isolate. Antimicrob. Agents Chemother. 41:2229-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metherall, J. E., H. Li, and K. Waugh. 1996. Role of multidrug resistance P-glycoproteins in cholesterol biosynthesis. J. Biol. Chem. 271:2634-2640. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki, Y., A. Geber, H. Miyazaki, D. Falconer, T. Parkinson, C. Hitchcock, B. Grimberg, K. Nyswaner, and J. E. Bennett. 1999. Cloning, sequencing, expression and allelic sequence diversity of ERG3 (c-5 sterol desaturase gene) in Candida albicans. Gene 236:43-51. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura, K., M. Niimi, K. Niimi, A. R. Holmes, J. E. Yates, A. Decottignies, B. C. Monk, A. Goffeau, and R. D. Cannon. 2001. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob. Agents Chemother. 45:3366-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolte, F. S., T. Parkinson, D. J. Falconer, S. Dix, J. Williams, C. Gilmore, R. Geller, and J. R. Wingard. 1997. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob. Agents Chemother. 41:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panwar, S. L., S. Krishnamurthy, V. Gupta, A.-M. Alarco, M. Raymond, D. Sanglard, and R. Prasad. 2001. CaALK8, an alkane assimilating cytochrome P450 confers multidrug resistance when expressed in a hypersensitive strain of Candida albicans. Yeast 18:1117-1129. [DOI] [PubMed] [Google Scholar]
- 39.Perepnikhatka, V., F. J. Fischer, M. Niimi, R. A. Baker, R. D. Cannon, Y.-K. Wang, F. Sherman, and E. Rustchenko. 1999. Specific chromosome alterations in fluconazole-resistant mutants of Candida albicans. J. Bacteriol. 181:4041-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pesti, M., J. M. Campbell, and J. F. Peberdy. 1981. Alteration of ergosterol content and chitin synthase activity in Candida albicans. Curr. Microbiol. 5:187-190. [Google Scholar]
- 41.Prasad, R., S. L. Panwar, and S. Krishnamurthy. 2002. Drug resistance mechanisms of human pathogenic fungi, p. 601-631. In R. A. Calderone and R. L. Cihlar (ed.), Fungal pathogenesis: principles and clinical applications. Marcel Dekker, Inc., New York, N.Y.
- 42.Prasad, R., S. L. Panwar, and Smriti. 2002. Drug resistance in yeasts—an emerging scenario. Adv. Microb. Physiol. 46:155-201. [DOI] [PubMed]
- 43.Prasad, R., P. D. Worgifosse, A. Goffeau, and E. Balzi. 1995. Molecular cloning and characterisation of a novel gene of C. albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27:320-329. [DOI] [PubMed] [Google Scholar]
- 44.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73-85. [DOI] [PubMed] [Google Scholar]
- 45.Sharom, F. J. 1996. The P-glycoprotein multidrug transporter: interactions with membrane lipids, and their modulation of activity. Biochem. Soc. Trans. 25:1088-1096. [DOI] [PubMed] [Google Scholar]
- 46.Sinicrope, F. A., P. K. Dudeja, B. M. Bissonnette, A. R. Safa, and T. A. Brasitus. 1992. Modulation of P-glycoprotein-mediated drug transport by alterations in lipid fluidity of rat liver canalicular membrane vesicles. J. Biol. Chem. 267:24995-25002. [PubMed] [Google Scholar]
- 47.Smriti, S. Krishnamurthy, and R. Prasad. 1999. Membrane fluidity affects functions of Cdr1p, a multidrug ABC transporter of Candida albicans. FEMS Microbiol. Lett. 173:475-481. [DOI] [PubMed] [Google Scholar]
- 48.Smriti, S. Krishnamurthy, V. Puri, B. L. Dixit, C. M. Gupta, and R. Prasad. 2002. ABC transporters Cdr1p, Cdr2p and Cdr3p of a human pathogen Candida albicans are general phospholipid translocators. Yeast 19:1-16. [DOI] [PubMed] [Google Scholar]
- 49.Swain, E., K. Baudry, J. Stukey, V. McDonough, M. Germann, and J. T. Nickels, Jr. 2002. Sterol-dependent regulation of sphingolipid metabolism in Saccharomyces cerevisiae. J. Biol. Chem. 277:26177-26184. [DOI] [PubMed] [Google Scholar]
- 50.Talibi, D., and M. Raymond. 1999. Isolation of a putative Candida albicans transcriptional regulator involved in pleiotropic drug resistance by functional complementation of a pdr1 pdr3 mutation in Saccharomyces cerevisiae. J. Bacteriol. 181:231-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Den Hazel, H. B., H. Pichler, M. A. Do Valle Matta, E. Leitner, A. Goffeau, and G. Daum. 1999. PDR16 and PDR17, two homologous genes of Saccharomyces cerevisiae, affect lipid biosynthesis and resistance to multiple drugs. J. Biol. Chem. 274:1934-1941. [DOI] [PubMed] [Google Scholar]
- 52.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zweytick, D., C. Hrastnik, S. D. Kohlwein, and G. Daum. 2000. Biochemical characterization and subcellular localization of the sterol C-24(28) reductase, Erg4p, from the yeast Saccharomyces cerevisiae. FEBS Lett. 470:83-87. [DOI] [PubMed] [Google Scholar]