Abstract
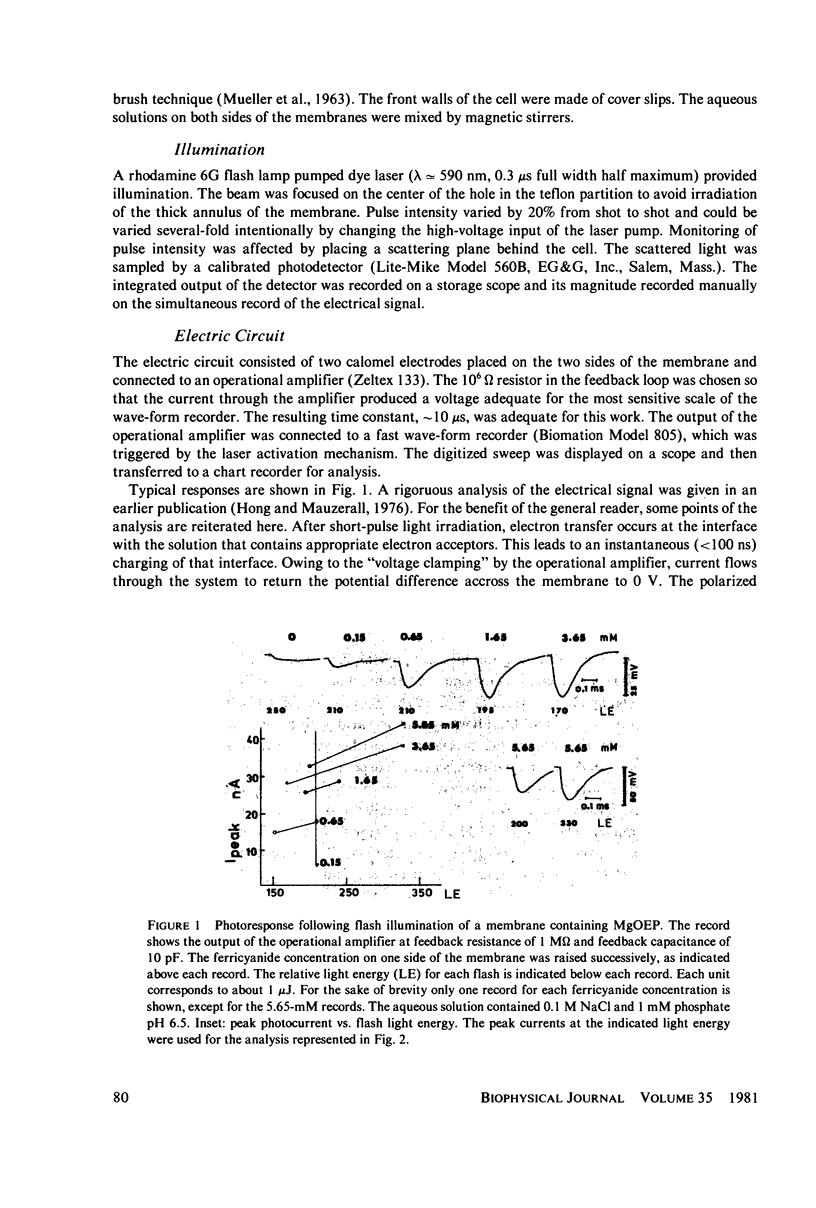
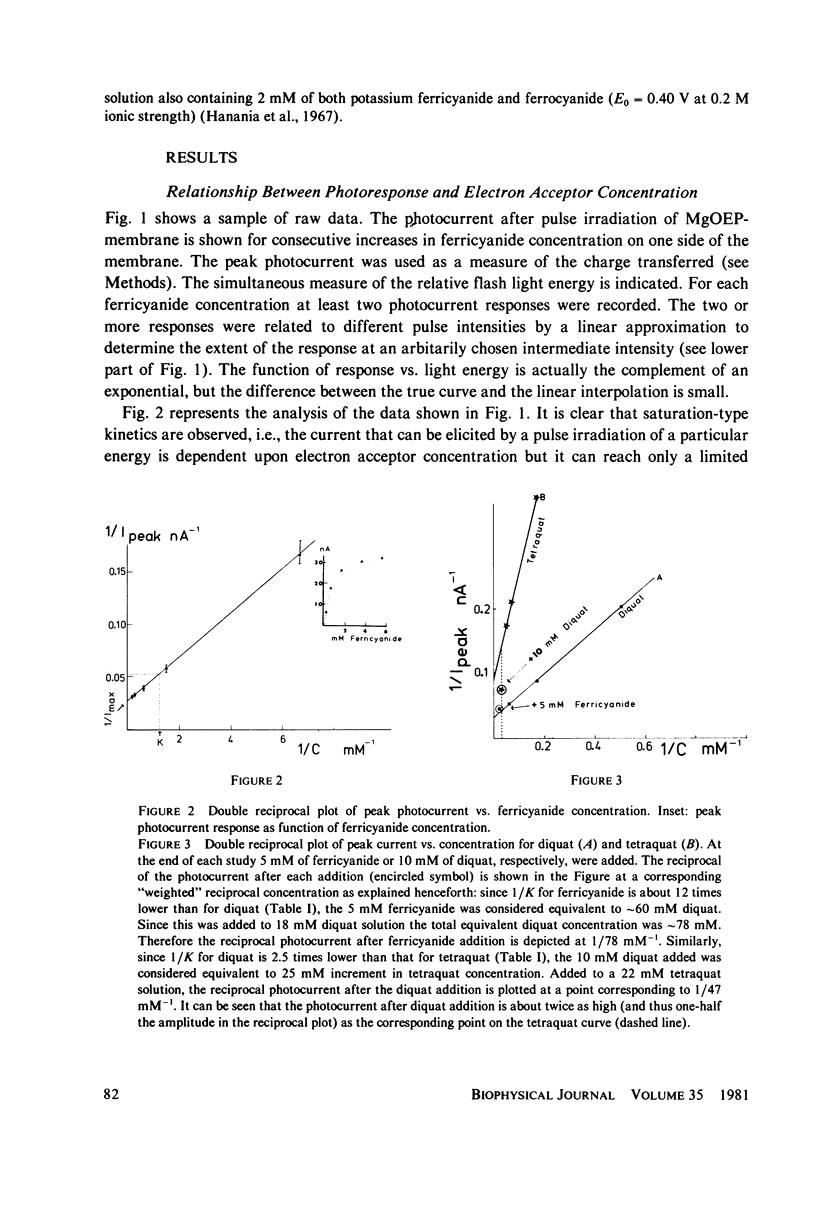
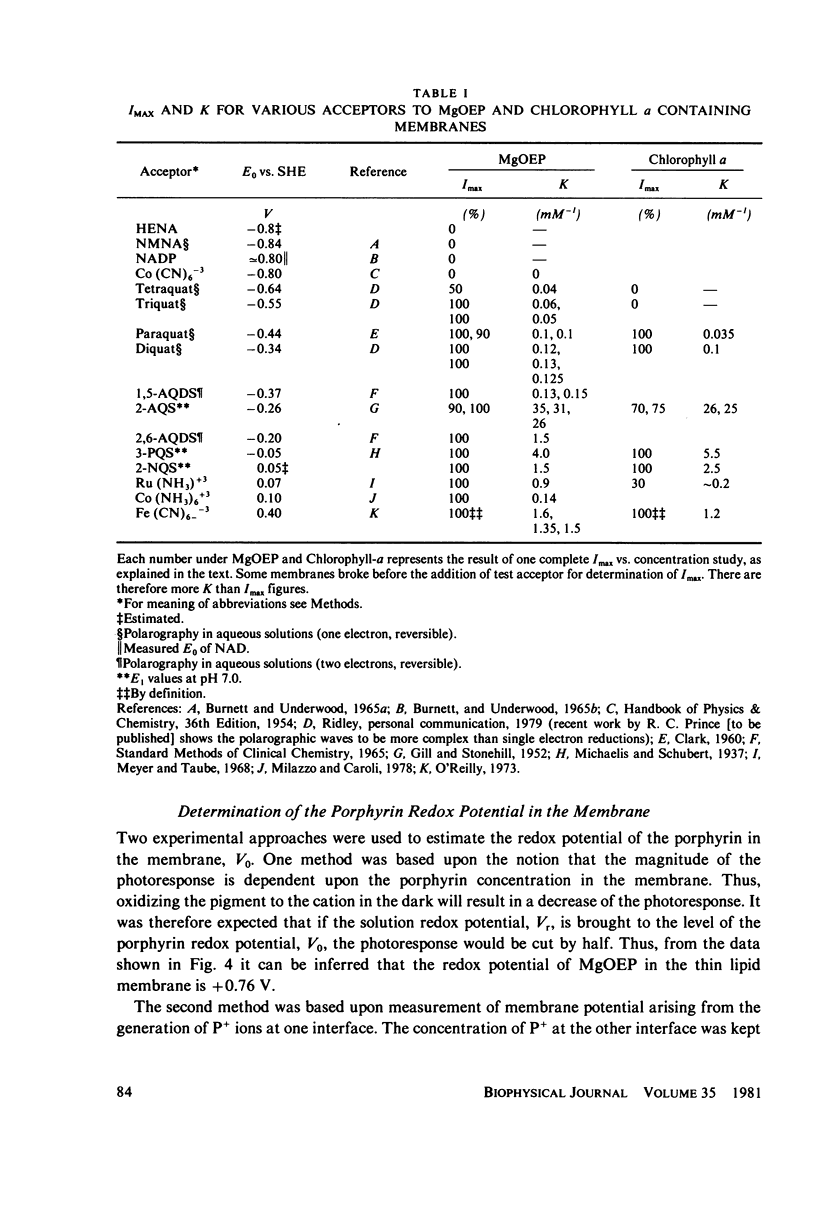
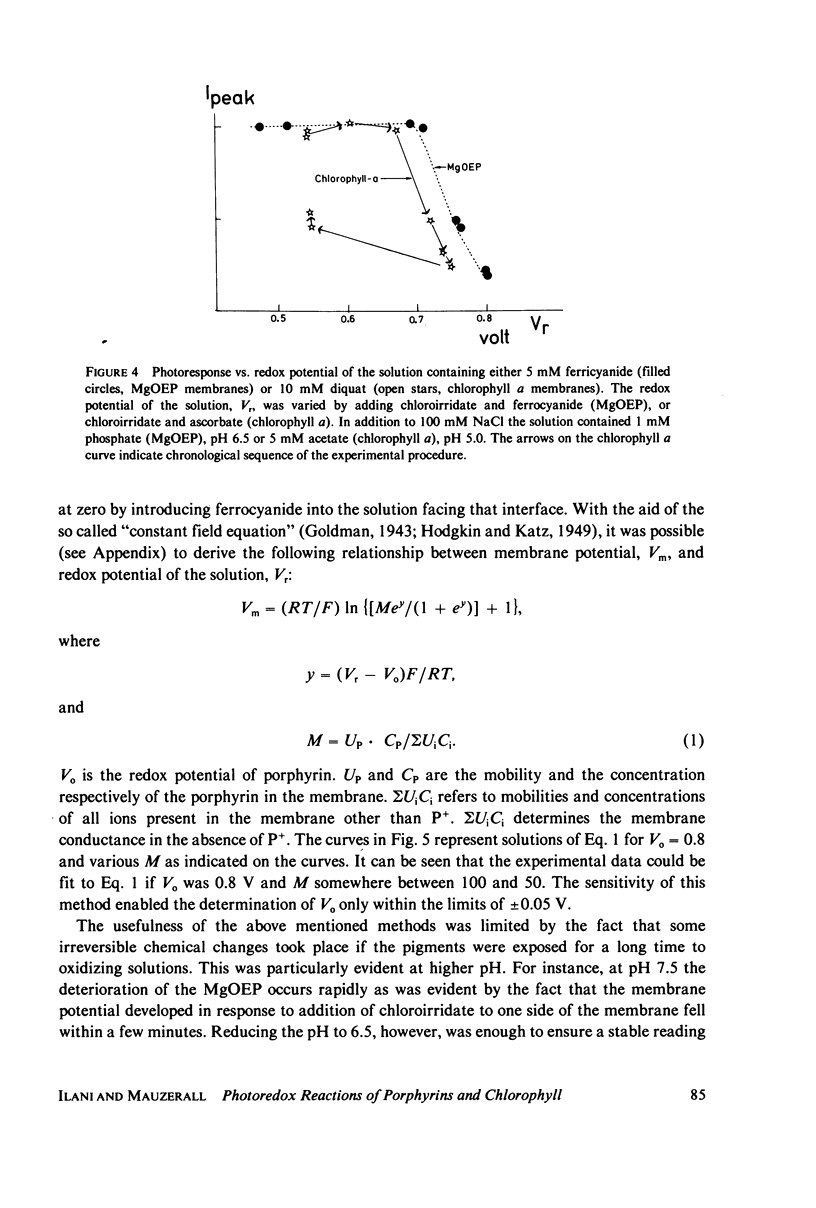
Lipid bilayers containing chlorophyll (Chl) or magnesium octaethylporphyrin (MgOEP) and separating solutions containing varying amounts of differing acceptors are illuminated by a dye laser pulse (FWHM 0.3 microseconds) at 590 mm. Interfacial charge transfer is measured at the first current peak in a voltage clamp circuit. The constants describing the hyperbolic saturations of the charge transferred by differing acceptors are only weakly related to the redox potential of the acceptors. An assymetric molecule, anthraquinone-2-sulfonate, is over 20 times as effective in accepting the electron as is the symmetrical anthraquinone-2,6-disulfonate. In contrast to this variable effectiveness, the maximum amount of charge transferred as a function of acceptor redox potential is constant up to a cut-off value: -0.6 V (vs. standard hydrogen electrode) for MgOEP and -0.5 V for Chl. The reversible redox potential of MgOEP in the bilayer was determined by following both the decrease in photoactivity and the transmembrane potential as a function of aqueous redox potential. It is +0.77 V for MgOEP and approximately 0.7 V for Chl (limited by stability). Thus, a total of 1.4 V of reversible redox potential (free energy) is obtained from 1.8 eV (internal energy) of the triplet excited state of MgOEP.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S., Finkelstein A., Katz I., Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol. 1976 Jun;67(6):749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Fuchs M. Potential energy barriers to ion transport within lipid bilayers. Studies with tetraphenylborate. Biophys J. 1975 Aug;15(8):795–830. doi: 10.1016/S0006-3495(75)85856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carapellucci P. A., Mauzerall D. Photosynthesis and porphyrin excited state redox reactions. Ann N Y Acad Sci. 1975 Apr 15;244:214–238. doi: 10.1111/j.1749-6632.1975.tb41533.x. [DOI] [PubMed] [Google Scholar]
- Fuhrhop J. H., Mauzerall D. The one-electron oxidation of metalloporphyrins. J Am Chem Soc. 1969 Jul 16;91(15):4174–4181. doi: 10.1021/ja01043a027. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanania G. I., Irvine D. H., Eaton W. A., George P. Thermodynamic aspects of the potassium hexacyanoferrate(3)-(2) system. II. Reduction potential. J Phys Chem. 1967 Jun;71(7):2022–2030. doi: 10.1021/j100866a008. [DOI] [PubMed] [Google Scholar]
- Hong F. T. Charge transfer across pigmented bilayer lipid membrane and its interfaces. Photochem Photobiol. 1976 Aug;24(2):155–189. doi: 10.1111/j.1751-1097.1976.tb06809.x. [DOI] [PubMed] [Google Scholar]
- Hong F. T., Mauzerall D. Photoemf at a single membrane-solution interface specific to lipid bilayers containing magnesium porphyrins. Nat New Biol. 1972 Nov 29;240(100):154–155. doi: 10.1038/newbio240154a0. [DOI] [PubMed] [Google Scholar]
- Krakover T., Ilani A., Mauzerall D. Apparent inhibition of photoredox reactions of magnesium octaethylporphyrin at the lipid bilayer-water interface by neutral quinones. Biophys J. 1981 Jul;35(1):93–97. doi: 10.1016/S0006-3495(81)84776-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly J. E. Oxidation-reduction potential of the ferro-ferricyanide system in buffer solutions. Biochim Biophys Acta. 1973 Apr 5;292(3):509–515. doi: 10.1016/0005-2728(73)90001-7. [DOI] [PubMed] [Google Scholar]
- Stamatoff J. B., Graddick W. F., Powers L., Moncton D. E. Direct observation of the hydrocarbon chain tilt angle in phospholipid bilayers. Biophys J. 1979 Feb;25(2 Pt 1):253–261. doi: 10.1016/s0006-3495(79)85289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


