Abstract
Seven Klebsiella pneumoniae and four Klebsiella oxytoca clinical isolates with different levels of resistance to ciprofloxacin were studied. Mutations in the topoisomerase genes were found in almost all strains, but the contribution of a multidrug efflux system homologous to AcrAB in Escherichia coli was also observed. Overexpression of this efflux system was demonstrated by immunoblotting with antibodies against E. coli AcrA.
In recent years, an increasing number of fluoroquinolone-resistant strains, especially of Escherichia coli, have been isolated from outpatients (3). Mutations in a small region at the beginning of the gyrA gene, called the quinolone resistance-determining region (QRDR), are often associated with the resistance phenotype. Recently, an additional mechanism of quinolone resistance, involving an active efflux system, has been found in E. coli strains with high levels of resistance and associated with both multiple mutations in the topoisomerase genes and overexpression of the AcrAB multidrug efflux system (2, 8, 11). This dual contribution to fluoroquinolone resistance has been demonstrated also for other organisms, such as Pseudomonas aeruginosa (4, 7), Staphylococcus aureus (6), and Streptococcus pneumoniae (5).
Resistance phenotypes are associated with mutations in the QRDRs of Klebsiella pneumoniae and Klebsiella oxytoca, too (1, 13), and the presence of the AcrAB operon in a clinical strain of K. pneumoniae was recently demonstrated (A. Domenech-Sanchez, S. Alberti, L. Martinez-Martinez, A. Pascual, I. Garcia, and V. J. Benedi, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-2018, p. 104, 2001), although its role in fluoroquinolone resistance was not discussed in this preliminary report.
Seven ciprofloxacin-resistant clinical strains of K. pneumoniae and four of K. oxytoca, isolated at the Verona University Hospital from August 1996 to October 1998, were studied. One ciprofloxacin-susceptible strain per species and E. coli AG100, which expresses normal levels of the AcrAB system (12), were also included. The MICs of novobiocin, ciprofloxacin, and nalidixic acid were tested by an agar dilution method, according to NCCLS guidelines (10), and the results are reported in Table 1. Novobiocin was included because it is a good substrate for the AcrAB efflux system of E. coli. The ciprofloxacin MICs for resistant strains ranged from 2 to 64 μg/ml, but only one strain (KLPN 86) showed a high level of resistance (MIC = 64 μg/ml). Sequencing of the QRDRs of gyrA and parC was carried out with all K. pneumoniae and K. oxytoca strains, by using conditions previously reported (1, 13). Mutations at codon 83 of gyrA in six of the seven resistant K. pneumoniae strains were found (Table 1). In five cases, the mutation at codon 83 was a C→A transversion in the codon TCC, resulting in the substitution of tyrosine for serine. In one strain, the mutation at codon 83 was a C→T transversion, resulting in a phenylalanine substitution for serine. Strain KLPN 105 showed the wild-type sequence. Mutations were also found in the QRDR region of parC in two resistant strains. In one case (strain KLPN 86) the mutation was at codon 80 and was a G→T transversion, resulting in the substitution of isoleucine for serine; in the other strain (KLPN 87) the mutation was at codon 79 and was an A→G transversion, resulting in the substitution of glycine for aspartic acid. In all resistant K. oxytoca strains, the mutation resulting in substitution of isoleucine for threonine at codon 83 was found in the gyrA gene. No mutations were found in the QRDRs of the gyrA and parC genes of the two ciprofloxacin-susceptible klebsiellae.
TABLE 1.
Antimicrobial susceptibilities, QRDR mutations, AcrA expression levels, and ciprofloxacin accumulation in clinical strains of K. pneumoniae and K. oxytoca
| Strain | MIC (μg/ml)
|
QRDR mutation
|
AcrA expression levelb (mean ± SD) | Level of ciprofloxacin accumulationc with- out CCCP | With CCCP | |||
|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin | Nalidixic acid | Novobiocin | gyrA | parC | ||||
| E. coli AG100a | 0.03 | 4 | 256 | None | None | 1.0b | 1.0c | 1.0c |
| K. pneumoniae | ||||||||
| KLPN 42 | 4 | >512 | 256 | S83Y | None | 1.0 ± 0.047 | 0.83 | 0.95 |
| KLPN 86 | 64 | >512 | >512 | S83F | S80I | 1.24 ± 0.051 | 0.42 | 1.08 |
| KLPN 87 | 4 | >512 | 16 | S83Y | D79G | 0.9 ± 0.07 | 0.85 | 1.1 |
| KLPN 90 | 4 | >512 | 128 | S83Y | None | 1.23 ± 0.03 | 0.55 | 1.1 |
| KLPN 94 | 0.03 | 4 | 64 | None | None | 0.9 ± 0.15 | 1 | 1.1 |
| KLPN 96 | 4 | >512 | 128 | S83Y | None | 1.31 ± 0.11 | 0.55 | 1.0 |
| KLPN 103 | 2 | >512 | 64 | S83Y | None | 1.0 ± 0.045 | 0.42 | 0.87 |
| KLPN 105 | 4 | >512 | 64 | None | None | 1.7 ± 0.15 | 0.35 | 0.83 |
| K. oxytoca | ||||||||
| KLOXY 106 | 4 | >512 | 256 | T83I | None | 2.2 ± 0.25 | 0.50 | 0.83 |
| KLOXY 108 | 2 | >512 | 256 | T83I | None | 2.2 ± 0.22 | 0.43 | 0.97 |
| KLOXY 109 | 2 | >512 | 256 | T83I | None | 1.65 ± 0.16 | 0.36 | 0.85 |
| KLOXY 114 | 0.03 | 4 | 16 | None | None | 0.53 ± 0.1 | 1.20 | 1.36 |
| KLOXY 116 | 4 | >512 | 128 | T83I | None | 1.14 ± 0.03 | 0.73 | 0.96 |
Wild-type strain that expresses a normal level of the AcrAB efflux system.
The levels of expression of the AcrA protein were determined as described in the text and were expressed relative to that for E. coli AG100. Tests were repeated three times.
The accumulation of ciprofloxacin (in nanograms per milligram of cells) was determined as described in the reference 9 and was expressed relative to that for E. coli AG100. CCCP was used at 100 μM.
To assess the presence of the AcrAB efflux system, acrA was amplified by using the primers KLACRA/FW (5′ ACTCGAGGTTTACAAATG) and KLACRA/R (5′ AGGCATGTCTTAACGGCT), derived from the published sequence (accession number AJ318073). The PCR conditions were 94°C for 1 min, 48°C for 1 min, and 72°C for 1 min for 30 cycles. The sequence of the 1.2-kb amplicons of the acrA gene from all isolates, both resistant and susceptible, was determined and compared with acrA of the deposited K. pneumoniae sequence (Domenech-Sanchez et al., 41st ICAAC). The acrA gene of our strains showed nucleotide identities ranging from 91.5 to 97.9% for K. pneumoniae and from 82 to 85.1% for K. oxytoca. The identities at the protein level ranged from 94.2 to 95.9% for K. pneumoniae and from 90.2 to 90.4% for K. oxytoca. In our strains, one amino acid (Q175) was always missing and most differences were found in a region of nine amino acids, from position 177 to position 185. In K. pneumoniae strains, other common differences from the deposited sequence were F92S, G103A, N153S, T162A, and V210L.
Using the available anti-AcrA antibodies (14), immunoblotting was employed to assess the correlation between fluoroquinolone resistance and expression of the AcrAB efflux system, according to the procedure already described (8). The strains were grown in Luria-Bertani broth at 37°C until the culture attained a density of about 0.2 mg (dry weight) ml−1. The cells were harvested by centrifugation and resuspended in the same volume of 1% sodium dodecyl sulfate, followed by heating in a boiling water bath for 10 min. Whole-cell extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% gel. Proteins were electroblotted onto nitrocellulose membranes and probed with polyclonal antibodies raised against E. coli AcrA, and the immunoblots were developed with the ECL Western blotting detection reagent (Amersham Pharmacia). The intensities of the bands obtained were compared by using ImageMaster VDS analysis software (Pharmacia Biotech). In each experiment, E. coli AG100, a reference strain with a normal level of expression, was included as a standard, and the AcrA levels in various strains were calculated as relative values in comparison with that for AG100. The experiments were repeated three times, and average values are reported in Table 1. Four of seven K. pneumoniae strains (including strain KLPN 105) showed overexpression of AcrA protein, at levels up to 130% of that of the control strain, E. coli AG100. The other three resistant K. pneumoniae strains and the susceptible strain of the same species showed AcrA levels ranging from 90 to 100% of that of the control. All K. oxytoca ciprofloxacin-resistant strains overproduced the AcrA protein, with levels ranging from 114 to 220% of that of the control. The susceptible strain of the same species had a reduced expression of AcrA (roughly 50% of that of AG100).
Ciprofloxacin accumulation in bacterial cells, in the presence or absence of the energy inhibitor carbonyl cyanide m-chlorophenylhydrazone (CCCP) was measured by the method of Mortimer and Piddock (9). A reduced ciprofloxacin accumulation, ranging from 15 to 65%, was found in all ciprofloxacin-resistant K. pneumoniae strains. This accumulation was reversed in all cases by addition of CCCP. In all cases, the ciprofloxacin-resistant K. oxytoca strains also showed a CCCP-reversible reduction (50% or less) of ciprofloxacin accumulation.
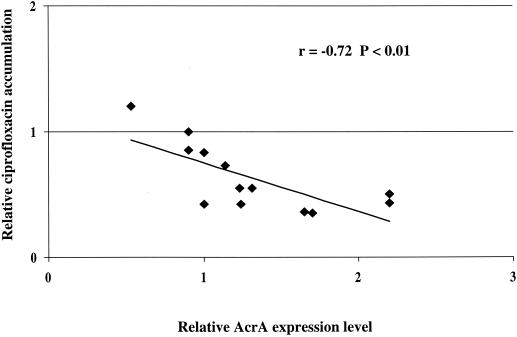
All strains of K. pneumoniae and K. oxytoca examined in our study contained sequences which showed a high degree of homology with the AcrA sequence of K. pneumoniae described previously, and a significant correlation (r = −0.72; P < 0.01) between AcrA expression and ciprofloxacin accumulation was observed (Fig. 1). The three resistant K. pneumoniae strains with normal expression of AcrA showed a lower uptake of ciprofloxacin than the reference strain, suggesting the possible involvement of efflux systems other than AcrAB. With only one exception (KLPN 105), resistance was also associated with mutations in the gyrA QRDR in all strains. Only two strains (KLPN 86 and KLPN 87) had mutations in the parC QRDR. All mutations were of previously described types (1, 13) except for D79G in parC of strain KLPN 87.
FIG. 1.
Correlation of AcrA expression and ciprofloxacin accumulation. The relative level of AcrA expression and the relative ciprofloxacin accumulation are reported in Table 1.
The contribution of efflux system overexpression to fluoroquinolone resistance was clearly demonstrated both by the behavior of strain KLPN 105, which did not carry any mutations in gyrA and parC but showed the highest level of AcrA expression, and by the two strains with mutations in both gyrA and parC, of which only the one overexpressing AcrA (KLPN 86) showed a high level of resistance.
Acknowledgments
This study was supported by grants from the Italian Ministry of the University and Scientific and Technological Research (MURST 40%, no. 2001068 775-003) and from the University of Verona (MURST 60%).
We are indebted to Hiroshi Nikaido for the generous gift of the anti-AcrA antibodies.
REFERENCES
- 1.Brisse, S., and J. Verhoef. 2001. Phylogenetic diversity of Klebsiella pneumoniae and Klebsiella oxytoca clinical isolates revealed by randomly amplified polymorphic DNA, gyrA and parC genes sequencing and automated ribotyping. Int. J. Syst. E vol. Microbiol. 51:915-924. [DOI] [PubMed] [Google Scholar]
- 2.Everett, M. J., Y. F. Jin, V. Ricci, and L. J. V. Piddock. 1996. Contributions of individual mechanisms to fluoroquinolone resistance in 36 Escherichia coli strains isolated from humans and animals. Antimicrob. Agents Chemother. 40:2380-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garau, J., M. Xercavins, M. Rodriguez-Carballeira, J. R. Gomez-Vera, I. Coll, D. Vidal, T. Llovet, and A. Ruiz-Bremon. 1999. Emergence and dissemination of quinolone-resistant Escherichia coli in the community. Antimicrob. Agents Chemother. 43:2736-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jalal, S., and B. Wretlind. 1998. Mechanisms of quinolone resistance in clinical strains of Pseudomonas aeruginosa. Microb. Drug Resist. 4:257-261. [DOI] [PubMed] [Google Scholar]
- 5.Jones, M. E., D. F. Sahm, N. Martin, S. Scheuring, P. Heisig, C. Thornsberry, K. Kohrer, and F. J. Schmitz. 2000. Prevalence of gyrA, gyrB, parC, and parE mutations in clinical isolates of Streptococcus pneumoniae with decreased susceptibilities to different fluoroquinolones and originating from worldwide surveillance studies during the 1997-1998 respiratory season. Antimicrob Agents Chemother. 44:462-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaatz, G. W., S. M. Seo, and C. A. Ruble. 1991. Mechanisms of fluoroquinolone resistance in Staphylococcus aureus. J. Infect. Dis. 163:1080-1086. [DOI] [PubMed] [Google Scholar]
- 7.Le Thomas, I., G. Couetdic, O. Clermont, N. Brahimi, P. Plesiat, and E. Bingen. 2001. In vivo selection of a target/efflux double mutant of Pseudomonas aeruginosa by ciprofloxacin therapy. J. Antimicrob. Chemother. 48:553-555. [DOI] [PubMed] [Google Scholar]
- 8.Mazzariol, A., Y. Tokue, T. M. Kanegawa, G. Cornaglia, and H. Nikaido. 2000. High-level fluoroquinolone-resistant clinical isolates of Escherichia coli overproduce multidrug efflux protein AcrA. Antimicrob. Agents Chemother. 44:3441-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortimer, P. G. S., and L. J. V. Piddock. 1991. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J. Antimicrob. Chemother. 28:639-653. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing: ninth informational supplement. National Committee for Clinical Laboratory Standards document M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.Oethinger, M., W. V. Kern, J. D. Goldman, and S. B. Levy. 1998. Association of organic solvent tolerance and fluoroquinolone resistance in clinical isolates of Escherichia coli. J. Antimicrob. Chemother. 41:111-114. [DOI] [PubMed] [Google Scholar]
- 12.Okusu, H., D. Ma, and H. Nikaido. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weigel, L. M., C. D. Steward, and F. C. Tenover. 1998. gyrA mutations associated with fluoroquinolone resistance in eight species of Enterobacteriaceae. Antimicrob. Agents Chemother. 42:2661-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zgurskaya, H. I., and H. Nikaido. 1999. AcrA is a highly asymmetric protein capable of spanning the periplasm. J. Mol. Biol. 285:409-420. [DOI] [PubMed] [Google Scholar]