Abstract
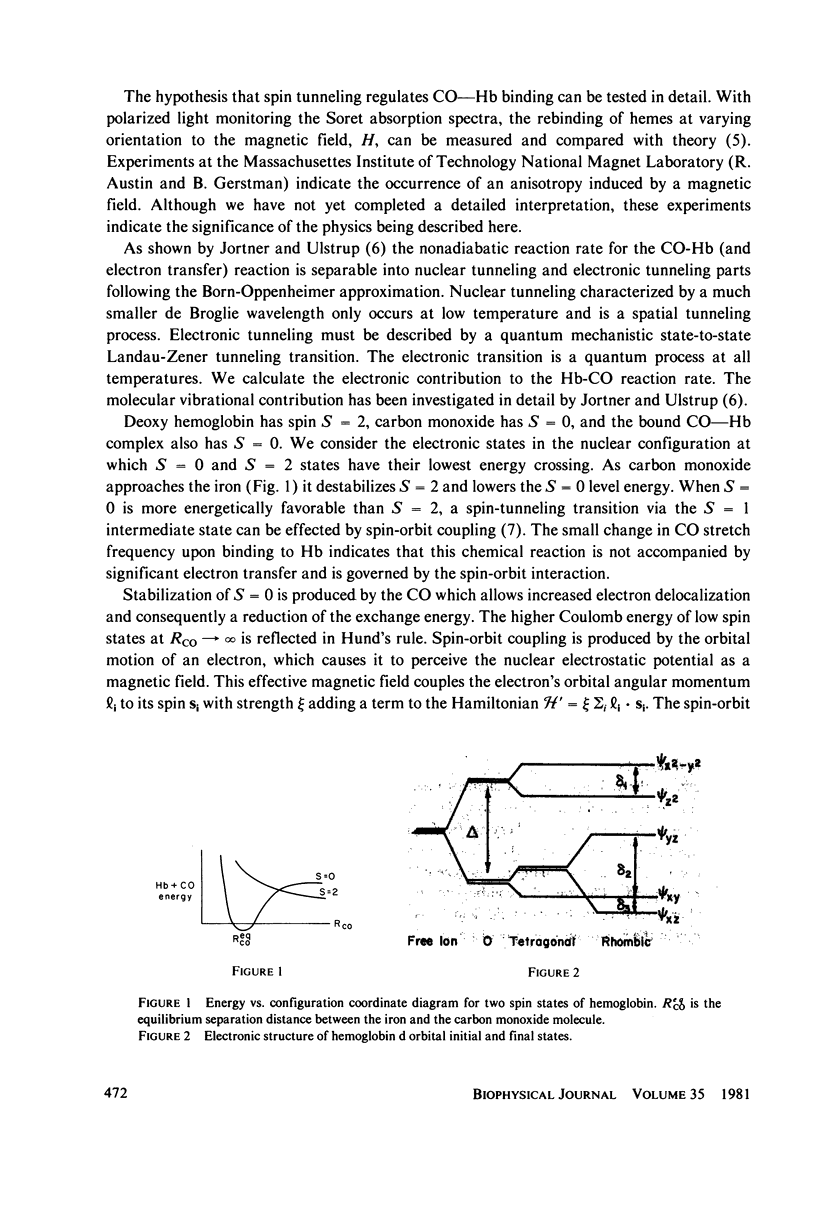
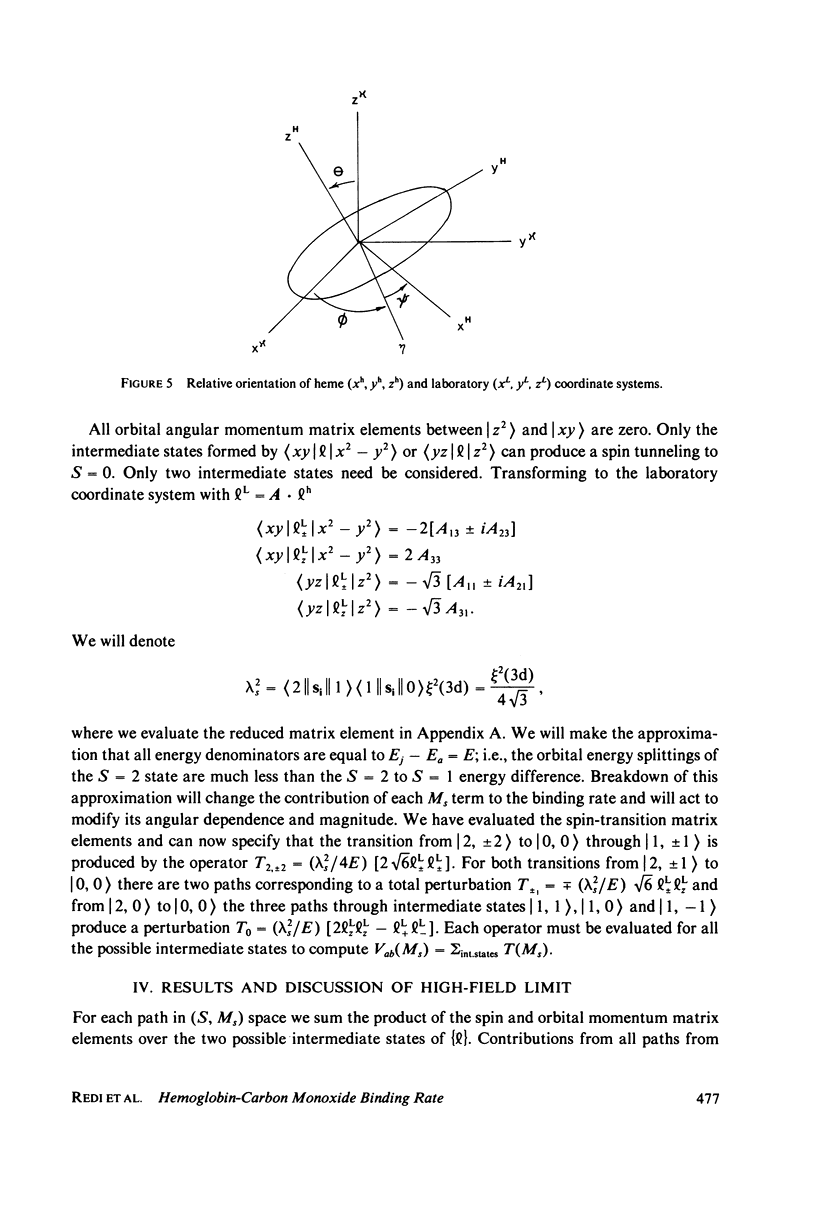
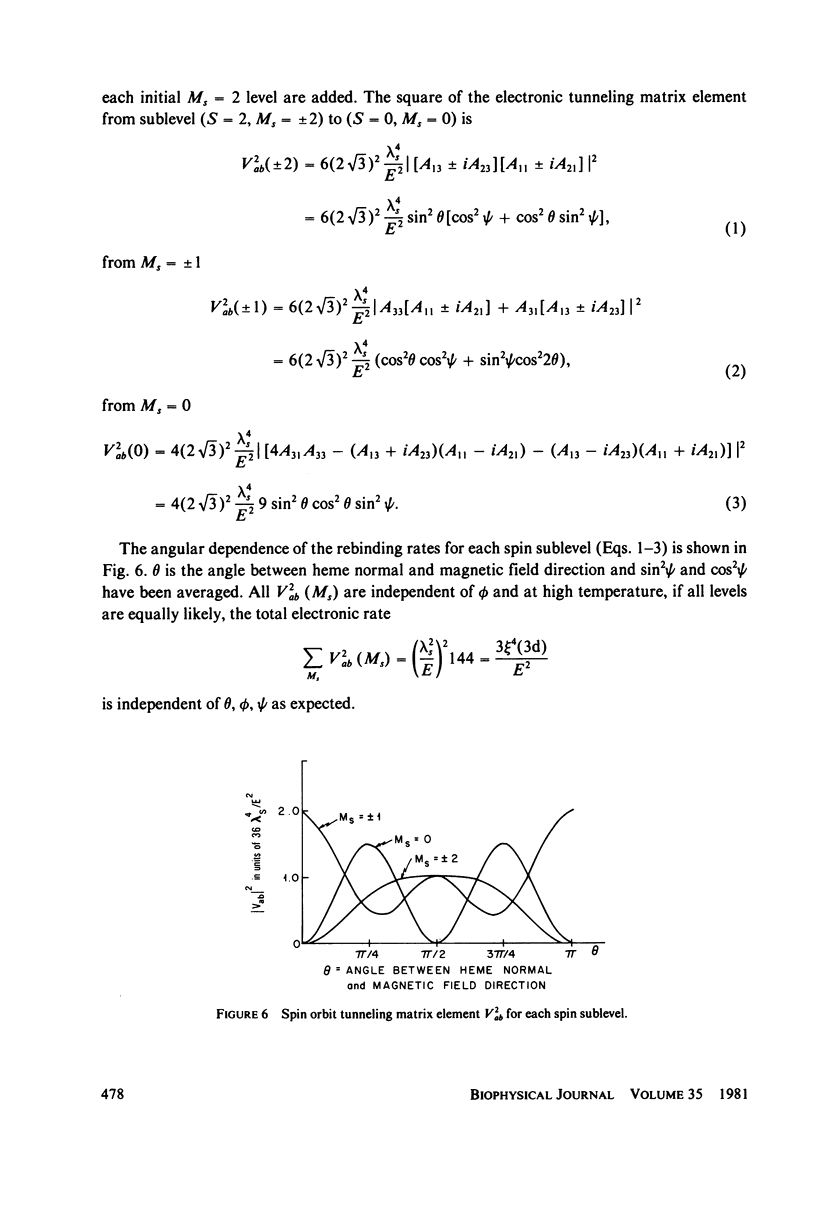
The spin-tunneling model of Hb--CO binding is used to calculate the binding rate at low temperature and high magnetic fields. The rate is calculated in second order perturbation theory assuming that spin-orbit coupling mediates the Hb iron electronic state change. The reaction which occurs at the crossing of the S = 2 and S = 0 energy vs. configuration coordinate curves is nonadiabatic, having a small electronic transition matrix element. Since detection of CO binding by polarized light in the Soret band makes it possible to observe hemes at specific orientation to the field direction, the rate is calculated for arbitrary heme orientation. Comparison with measurements at low temperature in zero field is made for spin quantization along the molecular crystal field direction.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hopfield J. J. Electron transfer between biological molecules by thermally activated tunneling. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3640–3644. doi: 10.1073/pnas.71.9.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew G. H., Kirchner R. F. Semiempirical calculations of model deoxyheme. Variation of calculated electromagnetic properties with electronic configuration and distance of iron from the plane. Biophys J. 1978 May;22(2):179–189. doi: 10.1016/S0006-3495(78)85483-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano N., Otsuka J., Tasaki A. Paramagnetic anisotropy measurements on a single crystal of deoxyhemoglobin. Biochim Biophys Acta. 1972 Sep 29;278(2):355–371. doi: 10.1016/0005-2795(72)90240-1. [DOI] [PubMed] [Google Scholar]
- Olafson B. D., Goddard W. A., 3rd Molecular description of dioxygen bonding in hemoglobin. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1315–1319. doi: 10.1073/pnas.74.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]


