Abstract
Linezolid is the first drug issued from the oxazolidinones, a novel class of antimicrobial agents with potent activity against gram-positive pathogens. A rabbit endocarditis model was used to compare the in vivo activities of different linezolid regimens mimicking intermittent dosing of 10 mg/kg of body weight every 12 h for 5 days or continuous (constant-rate) infusion of a daily dose of 20 mg/kg (for 5 days) or 40 mg/kg (for 3 and 5 days) and the activities of intermittent dosing and continuous infusion of vancomycin (for 5 days). The in vivo activities of these regimens were tested against three strains of methicillin-resistant Staphylococcus aureus. A human-like pharmacokinetic simulation was used for linezolid in order to improve the extrapolation of the results to human therapy. All linezolid regimens significantly reduced the numbers of S. aureus cells in aortic valve vegetations compared to the numbers in the control groups. Linezolid intermittent dosing had an in vivo bacteriostatic effect. Switching from intermittent dosing to continuous infusion (at the same dose) led to in vivo bactericidal activity, with a decrease of at least 3 log10 CFU/g of vegetation compared to the counts for the controls. After 5 days of treatment, continuous infusion of linezolid (corresponding to a daily dose of 40 mg/kg in humans) seemed to be at least as effective as vancomycin against the three strains. No resistant variant was isolated from the vegetations during any of the treatments. These data suggest that continuous infusion of linezolid could be an appropriate alternative to the use of glycopeptides for the treatment of severe methicillin-resistant S. aureus infections.
The increasing number of infections caused by resistant gram-positive bacteria has led to a search for new antimicrobial agents. Oxazolidinones, a novel class of synthetic antimicrobials, have been shown to have potent activities against gram-positive pathogens such as vancomycin-resistant enterococci, Streptococcus pneumoniae, coagulase-negative staphylococci, and Staphylococcus aureus (30). Linezolid, the first drug issued from this class, is active against gram-positive bacteria and displays nonbactericidal, time-dependent activity in vitro against staphylococci (15, 23). Oxazolidinones are bacterial protein synthesis inhibitors and act at a very early stage by preventing the formation of the initiation complex (2, 24, 25). This mechanism of action is specific to this class, and no cross-resistance with other antimicrobial agents has been observed. Linezolid has been approved by the Food and Drug Administration and is indicated for the treatment of infections in adult patients caused by gram-positive organisms (ZYVOX [linezolid] Advisory Committee Brochure, Pharmacia Upjohn, 2000). It can be administered intravenously (i.v.) or orally, and no dose adjustment is necessary when switching from the i.v. to the oral route of administration in humans.
An experimental endocarditis model allows the pharmacokinetic-pharmacodynamic aspects of therapeutic efficacy to be studied, and simulation of the pharmacokinetics in humans improves the analysis of in vivo activity and the extrapolation of results to human therapy (5). The present study used a rabbit endocarditis model to compare the in vivo activity of intermittent dosing versus that of continuous infusion of linezolid and vancomycin against three strains of S. aureus exhibiting different levels of methicillin resistance.
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 17 to 20 September 2000, and at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001.)
MATERIALS AND METHODS
Microorganisms.
Three methicillin-resistant S. aureus (MRSA) strains were studied according to the type of phenotypic expression of methicillin resistance (26). Two (strains SA-1 and SA-2) were clinical strains isolated from blood cultures, and the other (strain SA-3) was the S. aureus Col reference strain.
Antibiotics.
Linezolid and vancomycin were supplied by Pharmacia Upjohn (Kalamazoo, Mich.) and Lilly Company (Saint-Cloud, France), respectively.
Medium.
Mueller-Hinton (MH) broth (Sanofi Diagnostics Pasteur, Marne-la-Coquette, France) supplemented with calcium (25 mg/liter) and magnesium (12.5 mg/liter) was used for susceptibility tests and killing-curve experiments. Colony counts were determined with Trypticase soy agar (TSA) plates (Difco) and MH plates (Difco) for the time-kill experiments and the population analysis, respectively.
In vitro studies. (i) Determination of phenotypic classes in methicillin resistance.
The determination of phenotypic classes was based on an agar disk diffusion method, as described previously (10). Briefly, four antibiotic disks (with methicillin at 2.5, 15, and 100 μg and oxacillin at 30 μg) and three incubation temperatures (30, 35, and 40°C) were used to allow detection of temperature-related variations in phenotypic expression. To confirm this determination, population analysis was performed with the growth from overnight cultures of MRSA strains grown in MH broth. Serial dilutions were plated to obtain three different inocula (103, 106, and 109 CFU/ml) by using a spiral plater (Spiral System; Interscience, Saint-Nom-La-Breteche, France) on MH agar plates containing methicillin at concentrations ranging from 0.125 to 1,024 μg/ml. Bacterial counts were determined after 48 h of incubation at 37°C.
(ii) In vitro susceptibilities to antibiotics.
The MICs of linezolid and vancomycin for the three strains were determined in cation-supplemented MH broth by the microdilution technique (1). Overnight MH broth cultures were used to prepare inocula of 105 CFU/ml. The MICs of oxacillin were determined by the microdilution method in cation-supplemented MH broth containing 2% NaCl inoculated with 5 × 105 CFU/ml (16). Results were recorded at 24 h after incubation at 37°C. The MIC was defined as the lowest concentration of an antimicrobial agent that prevented turbidity after 24 h of incubation.
Time-kill experiments were performed in glass flasks containing MH broth (19) with an inoculum of 5 × 106 CFU/ml in the presence of linezolid or vancomycin at various concentrations (one, four, and eight times the MIC). One flask of inoculated cation-adjusted MH broth with no antibiotic served as the control. The surviving bacteria were counted after 0, 6, 24, and 48 h of incubation at 37°C by subculture of 50-μl serial dilutions of samples on TSA plates with a spiral plater. A bactericidal effect was defined as a decrease of ≥3 log10 CFU/ml compared to the initial inoculum after 24 h of incubation.
Pharmacokinetic studies.
A first step in the pharmacokinetic studies consisted of investigating the parameters allowing simulation of the kinetics of linezolid in human serum. Blood samples were taken from three healthy rabbits at 0, 2, 5, 10, 15, and 30 min and 1, 2, 4, 6, and 8 h after administration of an i.v. linezolid bolus of 10 mg/kg of body weight in order to determine spontaneous drug kinetics. The pharmacokinetic data were processed and compared with those for humans. A computer-controlled system was then used to obtain the human kinetic profiles for linezolid in rabbits (5). Simulation was intended to provide pharmacokinetic parameters close to those observed in healthy volunteers after administration of a single 600-mg bolus (ca. 10 mg/kg): mean half-life, 4.4 ± 2.4 h; peak concentration, 12.9 ± 1.6 mg/liter; and area under the curve, 80.2 ± 33.3 mg · h/liter (ZYVOX [linezolid] Advisory Committee Brochure). A total dose of 70 mg/kg needed to be infused into the rabbit over a 12-h period in order to simulate the kinetics in human serum after the administration of a 10-mg/kg dose. The infusion was delivered by a computer-controlled pump that allowed the flow to be adjusted to a profile mathematically defined in time. To validate the simulation, serum linezolid concentrations were determined in five rabbits after a single infusion and on the last day of treatment. In the second part of the study, the animals received linezolid as a continuous (constant-rate) i.v. infusion. The total daily dose used was that required to simulate the human kinetics of 10 mg/kg administered twice a day (b.i.d.), i.e., 140 mg/kg/24 h.
Vancomycin was administered as a constant-rate i.v. infusion (100 mg/kg/24 h) to obtain a serum steady-state concentration of about 20 to 25 mg/liter in the animals. For intermittent dosing the same dose (50 mg/kg) was administered to animals intramuscularly b.i.d. Samples were collected at various steady-state time points for each antibiotic treatment. Assays of serum were performed to determine peak and trough levels of vancomycin.
Endocarditis model.
The animals used for the endocarditis model were female New Zealand White rabbits (weight, 2.0 to 2.5 kg) housed in individual cages with free access to food and water. The procedures used in the experimental endocarditis model were as described previously (11, 21). To achieve valve injury, a polyethylene catheter was introduced into the left ventricle while the rabbits were under general anesthesia (intramuscular ketamine at 25 mg/kg). The catheter was left in place throughout the experiment. After catheterization for 24 h, each animal was inoculated i.v. with 1 ml of a bacterial solution (adjusted to 108 CFU/ml) with strain SA-1, SA-2, or SA-3. The animals were randomly assigned to no treatment (controls), treatment by intermittent dosing of vancomycin (at 50 mg/kg intramuscularly b.i.d.), treatment by continuous infusion of vancomycin (100 mg/kg/day), or a linezolid regimen mimicking intermittent dosing (10 mg/kg/12 h for 5 days) or continuous infusion (20 mg/kg/24 h for 5 days or 40 mg/kg/24 h for 3 and 5 days) in humans. Treatment was started 24 h after inoculation, and the antibiotics were administered to the animals via the marginal ear vein. The animals were killed by using an i.v. bolus of thiopental at the beginning of the treatment period (controls) or at the end of the 3- or 5-day regimen. Aortic valve vegetations were excised; immediately placed on ice; and then weighed, homogenized in 0.5 ml of saline buffer, and plated on TSA plates by using a spiral system. Dilutions of 10−1, 10−2, and 10−4 were used to eliminate potential carryover effects (mainly for groups treated by continuous infusion). Viable counts after 24 h of incubation at 37°C were expressed as the mean ± standard deviation log10 CFU per gram of vegetation. The lower detection limit for this method is 1 CFU per 50 μl of undiluted vegetation homogenate. To determine whether linezolid regimens could induce the selection of in vivo resistant variants, undiluted vegetation homogenates were spread on agar plates containing linezolid at concentrations corresponding to two- and fourfold the MIC. Bacterial counts were determined after 48 h of incubation at 37°C.
Antibiotic concentrations in serum.
Blood samples were obtained from animals through a catheter positioned in the median artery of the ear contralateral to the ear used for antibiotic infusion. Serum was frozen at −80°C until it was assayed. Linezolid was assayed by high-performance liquid chromatography (lower detection limit, 0.1 mg/liter; coefficient of variation, <10%) by a method adapted from that of Peng et al. (20). Vancomycin concentrations were determined by immunoenzymatic assay (lower detection limit, 2.5 mg/liter; coefficient of variation, 4.1 to 6.9%).
Statistics.
Statistical analysis was performed with StatView software (Abacus Concepts, Berkeley, Calif.). For each strain studied, analysis of variance was used to compare the effects between the different groups, followed by Scheffe's test to compare treated groups two by two. A P value ≤0.05 was considered significant.
RESULTS
Susceptibility tests.
The oxacillin MICs for SA-1, SA-2, and SA-3 were 16, 128, and >1,024 mg/liter, respectively. The MICs of linezolid and vancomycin for the three strains were 2 and 1 mg/liter, respectively. According to the determination of phenotypic classes of methicillin resistance (26), SA-1 and SA-2 exhibited heterogeneous low-level and high-level resistance, respectively, and SA-3 exhibited homogeneous resistance.
Time-kill curves.
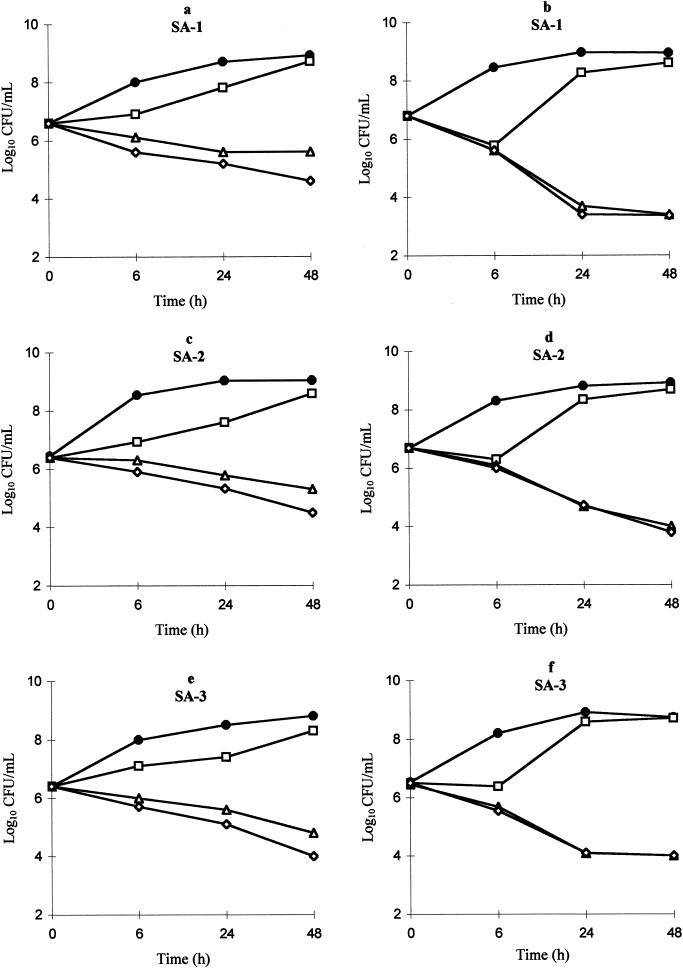
Linezolid showed only modest time-dependent activity against the three strains. At eight times the MIC, the decrease in the initial inoculum was close to 1 log after 24 h (Fig. 1a, c, and e). Vancomycin displayed bactericidal activity (a 2- to 3-log decrease after 24 h) against all strains (Fig. 1b, d, and f).
FIG. 1.
Killing curves for linezolid (a, c, and e) and vancomycin (b, d, and f) against SA-1, SA-2, and SA-3. •, control; □, one time the MIC; ▵, four times the MIC; ⋄, eight times the MIC.
Pharmacokinetic studies.
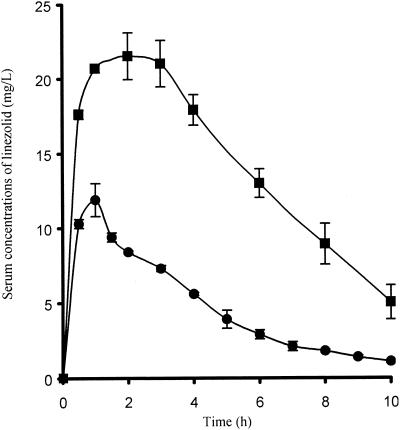
The serum linezolid levels obtained after administration of a dose simulating a 10-mg/kg dose in humans are shown in Fig. 2. The corresponding mean peak concentration, area under the curve, and half-life were 11.9 ± 1.1 mg/liter, 76.3 ± 5.9 mg · h/liter, and 2.7 ± 0.1 h, respectively, after administration of the first dose and 21.5 ± 1.3 mg/liter, 152.1 ± 9.2 mg · h/liter, and 3.4 ± 0.7 h, respectively, at day 5. Peak and trough vancomycin concentrations with intermittent dosing were 38.8 ± 6.2 and 12.3 ± 2.9 mg/liter, respectively. The concentrations of linezolid and vancomycin in serum on different days obtained with the continuous regimens are shown in Table 1.
FIG. 2.
Serum linezolid concentrations obtained after administration of a dose simulating a 10-mg/kg dose in humans: •, concentration obtained after administration of the first dose; ▪, concentration obtained at day 5. Error bars represent standard deviations.
TABLE 1.
Concentrations of linezolid or vancomycin (in serum) after continuous infusion
| Day | Concn (mg/liter) after administration of the following continuous infusion regimena:
|
||
|---|---|---|---|
| Linezolid
|
Vancomycin | ||
| 20 mg/kgb | 40 mg/kgc | ||
| 1 | 10.4 (1.6) | 27.1 (13.6) | ND |
| 2 | 19.4 (6.8) | 47.1 (18.8) | 22.3 (4.2) |
| 3 | 22.8 (4.2) | 49.7 (18.3) | ND |
| 5 | 32.5 (8.8) | 64.6 (6.3) | 25.1 (3.7) |
The values are means (standard deviations). ND, not done.
Simulated (daily) dose for humans.
Twice the recommended dose for humans.
Experimental endocarditis.
In vivo results are shown in Table 2. For all strains, the linezolid intermittent regimen failed to exhibit a bactericidal effect versus the effect of the control treatment, despite 5 days of treatment. Nevertheless, linezolid treatment reduced bacterial counts in vegetations significantly for SA-1, SA-2, and SA-3. Continuous infusion of linezolid appeared to be more efficient, with a ≥3 log10-CFU/g decrease of vegetations after 5 days for all regimens. Both vancomycin regimens (the continuous and intermittent i.v. regimens) displayed bactericidal activity against SA-2 and SA-3 but, surprisingly, not against SA-1, for which no decrease was observed, despite 5 days of therapy. None of the regimens studied after 48 h of incubation at 37°C showed S. aureus colonies on agar plates containing linezolid at two and four times the MIC.
TABLE 2.
Bacterial titers in vegetations
| Regimena | Mean ± SD log10 CFU/g of vegetation (no. of animals)
|
||
|---|---|---|---|
| Strain SA-1 | Strain SA-2 | Strain SA-3 | |
| Control | 8.8 ± 0.8 (14) | 9.0 ± 0.3 (8) | 9.1 ± 0.7 (9) |
| Linezolid ID, 5 days | 6.7 ± 1.3 (9)b | 7.3 ± 0.7 (9)b | 6.4 ± 1.0 (8)b |
| Vancomycin ID, 5 days | 8.8 ± 0.6 (5) | 2.9 ± 0.8 (5)c,d | 3.6 ± 1.2 (5)c |
| Linezolid CIV, 20 mg/kg, 5 days | 2.5 ± 0.2 (5)c,d | 4.8 ± 1.8 (6)c,d | 4.5 ± 1.7 (6)c |
| Linezolid CIV, 40 mg/kg, 3 days | 4.6 ± 2.1 (5)c | 4.4 ± 1.5 (6)c,d | 5.8 ± 2.4 (6)b |
| Linezolid CIV, 40 mg/kg, 5 days | 3.7 ± 1.1 (5)c,d | 2.5 ± 0.3 (5)c,d | 2.7 ± 0.5 (5)c,d |
| Vancomycin CIV, 30 mg/kg, 5 days | 8.5 ± 0.6 (5) | 2.8 ± 0.9 (5)c,d | 4.0 ± 0.9 (5)c |
ID, intermittent dosing simulating a human 10-mg/kg b.i.d. dose of linezolid or an intramuscular 50-mg/kg b.i.d. dose of vancomycin in humans; CIV, continuous (constant-rate) infusion simulating a 20- or 40-mg/kg daily dose of linezolid or a 30-mg/kg daily dose of vancomycin in humans.
P < 0.05 versus controls.
P < 0.001 versus controls.
P < 0.005 versus linezolid intermittent dosing at 5 days.
DISCUSSION
The experimental S. aureus endocarditis model has been used extensively to test the in vivo activities of new drugs or new regimens. It is particularly suitable for pharmacokinetic and pharmacodynamic analysis and the optimization of therapeutic efficacy. Computer-controlled simulation of human kinetic profiles of linezolid in rabbits was used in the present study to improve the analysis of the in vivo activity of this drug and the extrapolation of the results to human therapy (5). Owing to the very short spontaneous half-life of the drug in rabbits (30 min; unpublished data), simulation was required to reach valid conclusions relative to human applications. A twice-daily 600-mg dose of linezolid is recommended in clinical practice for the treatment of patients with infections due to MRSA (ZYVOX [linezolid] Advisory Committee Brochure). To our knowledge, no published experimental study has used the i.v. form of linezolid. Bioavailability is 100% with i.v. administration in rabbits, but it is only about 30% with the oral form (18).
The first part of this study evaluated the therapeutic efficacy of recommended intermittent dosing of linezolid for 5 days. The bacterial counts in aortic valve vegetations from rabbits treated with linezolid were significantly reduced for all strains, but the drug failed to exhibit bactericidal activity, despite 5 days of treatment. Two previous studies investigated the efficacy of oral linezolid at different doses in an endocarditis model involving two S. aureus strains, one susceptible to methicillin (18) and the other resistant to methicillin (8). Linezolid (given orally three times a day) displayed in vivo bactericidal activity when it was given as 50- and 75-mg/kg doses three times a day, but only the high-dose regimen (75 mg/kg three times a day) was as efficient as vancomycin in terms of the number of surviving bacteria in the vegetations. The total daily doses used (50 mg/kg three times a day = 150 mg/kg and 75 mg/kg three times a day = 225 mg/kg) were close to those used in our study (70 mg/kg b.i.d. = 140 mg/kg and 140 mg/kg b.i.d. = 280 mg/kg), but the drug distribution differed. It is likely that administration by the oral route three times a day gave a kinetic profile close to that of continuous delivery. This may account (at least in part) for the good results observed, which were similar to ours with the continuous infusion regimen.
The pharmacokinetic and pharmacodynamic aspects of this study probably explain the difference in the in vivo activities between the regimens used. In fact, trough levels of linezolid were less than the MIC on the first day of dosing (intermittent dosing) and close to twice the MIC on day 5. Moreover, concentrations in serum fell under 8 mg/liter (i.e., four times the MIC) as early as the third hour on day 1 and after the eighth hour on day 5. Thus, the concentration in serum was less than four times the MIC for about 75% of the time on day 1 and 33% of the time on day 5. The accumulation of the drug between day 1 and day 5 has already been described by Dailey et al. (8) and from the clinical use of linezolid in two patients with pancreatic abscesses (13). For antibiotics whose activities are time dependent, the time above the MIC (T > MIC) is usually considered a critical parameter in the assessment of therapeutic efficacy (7). In particular, continuous infusion of β-lactams has been studied. In vivo and in vitro studies have shown that, for the same daily dose, continuous infusion of ceftazidime improves the T > MIC compared to that obtained by intermittent dosing (9, 17, 22). The maximal activity of continuous infusion was obtained at a steady-state concentration in serum equal to a multiple of the MIC. For example, the activity of ceftazidime is now considered optimal when the concentration is four to eight times the MIC (6, 29). Continuous infusion of linezolid was used to investigate whether it results in a possible improvement in the in vivo effect and to obtain a valid comparison of linezolid and vancomycin. By use of the same total daily dose used for the intermittent dosing regimen, the steady-state concentration in serum increased from 10.4 ± 1.6 mg/liter on day 1 to 32.5 ± 8.8 mg/liter on day 5, confirming the accumulation previously described with the intermittent dosing regimen. Thus, continuous infusion provided a T > MIC of 100% and a ratio between the steady-state concentration in serum and the MIC of 5 to 16 over 5 days of treatment. Although linezolid was nonbactericidal in vitro, continuous infusion showed bactericidal activity in vivo. The endocarditis model is considered to require bactericidal drugs to achieve efficacy in vivo. However, other host factors (known or unknown) could be involved in the successful treatment of severe infections such as endocarditis and might intensify the in vivo activity of linezolid (4). Further studies are needed to identify and evaluate the possible impacts of these factors as well as the penetration of linezolid within aortic vegetations. Moreover, an in vitro experiment (C. G. Gemmell and C. W. Ford, 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1537, 1999) has shown that linezolid inhibits the expression of S. aureus virulence factors such as α-hemolysin, δ-hemolysin, and coagulase. This reduction of bacterial virulence factor expression could increase the in vivo activity of linezolid.
To evaluate the effects of higher doses of linezolid, continuous infusion was performed, in which the total dose was doubled for 3 and 5 days. Steady-state concentrations in serum were increased, with ratios of the steady-state concentration in serum to the MIC of 13.5, 25, and 32 for days 1, 3, and 5, respectively. The results indicate that this higher dose was beneficial, with a significant bactericidal effect detected at as early as 3 days of treatment and an increase in bactericidal intensity detected on day 5.
No linezolid-resistant variants were isolated from vegetations during the linezolid treatments. The administration route (intermittent dosing or continuous infusion) and the dosage (20- or 40-mg/kg daily dose) used did not appear to induce the development of resistance in the surviving bacteria at the end of therapy. However, the treatment period (3 and 5 days) was probably too short to detect a possible induction of resistance, despite a steady-state concentration in serum close to 25 times the MIC for the continuous infusion group. Zurenko et al. (30) reported a low spontaneous mutation frequency in vitro (<8 × 10−11) in S. aureus by use of two, four, and eight times the MIC of linezolid; and a rapid development of resistance was not observed. The length of therapy seems to be a factor more important for the development of resistance than the dose used. According to clinical reports, linezolid resistance develops mainly in Enterococcus species (12), with the observation of linezolid resistance in an S. aureus strain being reported only once (27). Patients were treated for at least 3 weeks before linezolid resistance was reported. Despite the low level of mutation frequency in vitro and the absence of resistance development in our study, combination therapy should be investigated as a means of limiting the emergence of resistance from the clinical use of linezolid.
Recent studies have reported on the hematologic toxicity associated with linezolid therapy. Thrombocytopenia (3, 28) and myelosuppression (14) have been described and correlated with the length of treatment (>10 to 14 days). The manufacturer recommends that platelet counts be monitored in patients who have preexisting thrombocytopenia, those who are at increased risk of bleeding or who are receiving other concomitant medications that may decrease the platelet counts, and those who have been receiving linezolid therapy for >2 weeks. These adverse events are reversible, and hematologic parameters returned to normal after the discontinuation of linezolid therapy. To our knowledge, no data are available on the impact of high serum linezolid levels on drug toxicity. Although an enhancement of hematologic effects could be suspected with increased linezolid levels, studies are needed to specify the role of either the dose or the length of treatment on the hematologic toxicity of linezolid. Furthermore, human pharmacokinetic studies on the continuous infusion of linezolid could be of interest in order to compare the levels in serum obtained in our model and in humans and to specify the impact of this mode of administration on the accumulation of linezolid in serum over the course of therapy.
In our study, no benefit of continuous infusion of vancomycin over intermittent dosing in terms of activity against the three MRSA strains tested was observed. The in vitro characteristics (MIC, time-kill curves, etc.) of the three MRSA strains studied were similar, which makes it difficult to explain the lack of in vivo activity of vancomycin against SA-1. Further studies are in progress to investigate the failure of vancomycin therapy against this strain.
Conclusion.
Continuous infusion of linezolid produced bactericidal activity in vivo after 5 days of treatment, whereas in vitro tests had predicted only a bacteriostatic effect. Linezolid activity seems to depend mainly on (i) T > MIC and (ii) the ratio of the steady-state concentration in serum to the MIC.
These data suggest that the in vivo activity of linezolid could be improved by continuous infusion. Further studies are needed to investigate the potential clinical benefit of continuous infusion. The use of this administration mode could be an appropriate alternative to the use of glycopeptides for the treatment of severe MRSA infections.
Combination therapy should also be investigated as a means of increasing the early in vivo activity of linezolid and protecting the future of a promising new drug.
Acknowledgments
We are grateful to Anne-Françoise Miègeville and Virginie Le Mabecque for excellent technical assistance.
This work was supported by a grant from Pharmacia Upjohn, Kalamazoo, Mich.
REFERENCES
- 1.Amsterdam, D. 1996. Susceptibility testing of antibiotics in liquid media, p. 52-111. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 2.Aoki, H., L. Ke, S. M. Poppe, T. J. Poel, E. A. Weaver, R. C. Gadwood, R. C. Thomas, D. L. Shinabarger, and M. C. Ganoza. 2002. Oxazolidinone antibiotics target the P site on Escherichia coli ribosomes. Antimicrob. Agents Chemother. 46:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attassi, K., E. Hershberger, R. Alam, and M. J. Zervos. 2002. Thrombocytopenia associated with linezolid therapy. Clin. Infect. Dis. 34:695-698. [DOI] [PubMed] [Google Scholar]
- 4.Bayer, A. S., D. Cheng, M. R. Yeaman, G. R. Corey, R. S. McClelland, L. J. Harrel, and V. G. Fowler. 1998. In vitro resistance to thrombin-induced platelet microbicidal protein among clinical bacteremic isolates of Staphylococcus aureus correlates with an endovascular infectious source. Antimicrob. Agents Chemother. 42:3169-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugnon, D., G. Potel, J. Caillon, D. Baron, H. B. Drugeon, P. Feigel, and M. F. Kergueris. 1998. In vivo simulation of human pharmacokinetics in the rabbit. Bull. Math. Biol. 60:545-567. [DOI] [PubMed] [Google Scholar]
- 6.Cappelletty, D. M., S. L. Kang, S. M. Palmer, and M. J. Rybak. 1995. Pharmacodynamics of ceftazidime administered as continuous infusion or intermittent bolus alone and in combination with single daily-dose amikacin against Pseudomonas aeruginosa in an in vitro infection model. Antimicrob. Agents Chemother. 39:1797-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbon, C. 1990. Impact of the antibiotic schedule on efficacy in experimental endocarditis. Scand. J. Infect. Dis. Suppl. 74:163-172. [PubMed] [Google Scholar]
- 8.Dailey, C. F., C. L. Dileto-Fang, L. V. Buchanan, M. P. Oramas-Shirey, D. H. Batts, C. W. Ford, and J. K. Gibson. 2001. Efficacy of linezolid in treatment of experimental endocarditis caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:2304-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David, T. J., and J. Devlin. 1989. Continuous infusion of ceftazidime in cystic fibrosis. Lancet i:1454-1455. [DOI] [PubMed]
- 10.Drugeon, H. B., and V. Drocourt. 1994. Méthode simple pour déterminer la classe d'expression de la résistance à la méticilline. Path. Biol. 42:460-464. [PubMed] [Google Scholar]
- 11.Durack, D. T., and P. B. Beeson. 1972. Experimental bacterial endocarditis. II. Survival of bacteria in endocardial vegetations. Br. J. Exp. Pathol. 53:50-53. [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. [DOI] [PubMed] [Google Scholar]
- 13.Gopal Rao, G., A. Steger, and C. M. Tobin. 2001. Linezolid levels in pancreatic secretions. J. Antimicrob. Chemother. 48:931-932. [DOI] [PubMed] [Google Scholar]
- 14.Green, S. L., J. C. Maddox, and E. D. Huttenbach. 2001. Linezolid and reversible myelosuppression. JAMA 285:1291. [DOI] [PubMed] [Google Scholar]
- 15.Kaatz, G. W., and S. M. Seo. 1996. In vitro activities of oxazolidinone compounds U100592 and U100766 against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 40:799-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 17.Nicolau, D. P., C. H. Nightingale, M. A. Banevicius, Q. Fu, and R. Quintiliani. 1996. Serum bactericidal activity of ceftazidime: continuous infusion versus intermittent injections. Antimicrob. Agents Chemother. 40:61-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oramas-Shirey, M. P., L. V. Buchanan, C. L. Dileto-Fang, C. F. Dailey, C. W. Ford, D. H. Batts, and J. K. Gibson. 2001. Efficacy of linezolid in a staphylococcal endocarditis rabbit model. J. Antimicrob. Chemother. 47:349-352. [DOI] [PubMed] [Google Scholar]
- 19.Pearson, R. D., R. T. Steigbigel, H. T. Davis, and S. W. Chapmann. 1980. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob. Agents Chemother. 18:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng, G. W., R. P. Stryd, S. Murata, M. Igarashi, K. Chiba, H. Aoyama, M. Aoyama, T. Zenki, and N. Ozawa. 1999. Determination of linezolid in plasma by reversed-phase high-performance liquid chromatography. J. Pharm. Biomed. Anal. 20:65-73. [DOI] [PubMed] [Google Scholar]
- 21.Perlman, B. B., and L. R. Freedman. 1971. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J. Biol. Med. 44:206-213. [PMC free article] [PubMed] [Google Scholar]
- 22.Robaux, M. A., L. Dube, J. Caillon, D. Bugnon, M. F. Kergueris, D. Navas, P. Le Conte, D. Baron, and G. Potel. 2001. In vivo efficacy of continuous infusion versus intermittent dosing of ceftazidime alone or in combination with amikacin relative to human kinetic profiles in a Pseudomonas aeruginosa rabbit endocarditis model. J. Antimicrob. Chemother. 47:617-622. [DOI] [PubMed] [Google Scholar]
- 23.Rybak, M. J., D. M. Cappelletty, T. Moldovan, J. R. Aeschlimann, and G. W. Kaatz. 1998. Comparative in vitro activities and postantibiotic effects of the oxazolidinone compounds eperezolid (PNU-100592) and linezolid (PNU-100766) versus vancomycin against Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus faecalis, and Enterococcus faecium. Antimicrob. Agents Chemother. 42:721-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinabarger, D. L., K. R. Marotti, R. W. Murray, A. H. Lin, E. P. Melchior, S. M. Swaney, D. S. Dunyak, W. F. Demyan, and J. M. Buysse. 1997. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob. Agents Chemother. 41:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swaney, S. M., H. Aoki, M. C. Ganoza, and D. L. Shinabarger. 1998. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob. Agents Chemother. 42:3251-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasz, A., S. Nachman, and H. Leaf. 1991. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 35:124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, and R. C. Moellering, Jr. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]
- 28.Waldrep, T. W., and D. J. Skiest. 2002. Linezolid-induced anemia and thrombocytopenia. Pharmacotherapy 22:109-112. [DOI] [PubMed] [Google Scholar]
- 29.Xiong, Y. Q., J. Caillon, X. Y. Zhou, G. Potel, D. Bugnon, P. Le Conte, F. Le Gallou, R. Le Floch, D. Baron, and H. Drugeon. 1995. Treatment of experimental rabbit infective endocarditis due to a multidrug-resistant Pseudomonas aeruginosa with high-dose ceftazidime alone and combined with amikacin or sulbactam or both. J. Antimicrob. Chemother. 35:697-706. [DOI] [PubMed] [Google Scholar]
- 30.Zurenko, G. E., B. H. Yagi, R. D. Schaadt, J. W. Allison, J. O. Kilburn, S. E. Glickman, D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]