Abstract
Artesunate (ARS) is a water-soluble artemisinin derivative that is a potential alternative to quinine for the treatment of severe childhood malaria. We studied the pharmacokinetics and bioavailability of ARS given by the intramuscular (i.m.) route in an open crossover study design. Fourteen children were randomized to receive intravenous (i.v.) ARS in a loading dose (2.4 mg/kg of body weight) followed 12 h later by an i.m. dose (1.2 mg/kg) (group I), and 14 children were randomized to receive i.m. ARS (2.4 mg/kg) followed by an i.v. dose of ARS (1.2 mg/kg) (group II). We carried out a two-compartment analysis of ARS and dihydroartemisinin (DHA; the principal antimalarial metabolite) levels in 21 children (groups I and II combined). Absorption of i.m. ARS was rapid, with the maximum concentration of DHA in serum being achieved in less than 1 h in most children (median time to the maximum concentration of drug in serum, 35.1 min; range, 10.8 to 71.9 min). The absolute bioavailability of DHA was a median of 86.4% (range, 11.4 to 462.1%), the median steady-state volume of distribution was 1.3 liters/kg (range, 0.5 to 7.9 liters/kg), and the median clearance was 0.028 liters/kg/min (range, 0.001 to 1.58 liters/kg/min). There were no major adverse events attributable to ARS. Parasite clearance kinetics were comparable between the two treatment groups. These results support the use of i.m. ARS in children with severe malaria.
Plasmodium falciparum malaria kills more than 2 million children in sub-Saharan Africa annually. Chloroquine resistance is now established in Africa, leaving quinine as the first choice for the treatment of severe malaria (17). Even with quinine treatment, severe malaria is associated with a mortality rate of ∼20% in hospitalized children (11). Quinine causes hyperinsulinemic hypoglycemia in a significant proportion of patients, sometimes despite glucose supplementation (1, 7). There are also other potential hazards from the use of quinine. If intravenous (i.v.) quinine is given too rapidly, it may cause prolongation of the QTc interval and predispose the individual to polymorphic ventricular arrhythmias (8). In many hospitals reliable monitoring of quinine infusion rates is not possible because resources are lacking. The intramuscular (i.m.) route for quinine administration has recently regained favor. It reduces the risk of dosing errors that can cause cardiotoxicity, although the risk of hypoglycemia is unaltered. For these reasons, there is an urgent need to develop alternative safe antimalarials that are efficacious in patients with severe malaria.
Artemisinin derivatives are obtained from Artemisia annua (sweet wormwood) and make up a rapidly acting class of antimalarials that can be used to treat severe malaria. Large prospective trials of i.m. artemether (an oil-soluble artemisinin derivative) for treatment of severe malaria have been conducted with adults and children (4, 9, 16). Pharmacokinetic analyses suggest that absorption of artemether may be poor and erratic, although overall the efficacy of artemether was similar to that of quinine in a large randomized comparison conducted in The Gambia (16). The lack of an i.v. formulation for artemether, however, precludes formal bioavailability assessments.
Artesunate (ARS) is a water-soluble hemisuccinate semisynthetic derivative of artemisinin that is one of the most rapidly acting antimalarials available (2). It can be admininstered by the i.v., i.m., intrarectal, and oral routes, providing an opportunity to carry out bioavailability studies with patients with malaria of various degrees of severity. The pharmacokinetics of ARS (i.v., oral, and intrarectal) have only been studied in children with uncomplicated and moderate malaria (6, 12). We have therefore carried out the first assessment of the bioavailability, efficacy, and toxicity of i.m. ARS in Gabonese children with severe malaria to determine its potential usefulness in this population. This is the population that is at the highest risk of death from malaria and that will therefore benefit most from an alternative to quinine.
(This work was conducted by T. Planche in partial fulfillment of the requirements for an M.D. degree from the University of London.)
MATERIALS AND METHODS
Study design.
This was an open, randomized, crossover study comparing i.v. and i.m. ARS. The study was conducted in the emergency room and the general pediatric ward at Central Hospital, Libreville, Gabon, from December 2000 to March 2001. The study was approved by the Ethics Committees of the International Foundation of the Albert Schweitzer Hospital (Lambaréné, Gabon), the Gabonese Ministry of Health (Libreville, Gabon), and the Medical Faculty of the University of Tübingen (Tübingen, Germany). A computer-generated randomization list (in permuted blocks of eight patients) was used to assign a treatment category and was supplied in an opaque sealed envelope that was opened only when the patient entered the study. Treatment categories were as follows: group I, i.v. ARS at 2.4 mg/kg (6.24 μmol/kg) of body weight, followed 12 h later by an i.m. injection at 1.2 mg/kg (3.12 μmol/kg) of body weight; group II, i.m. ARS at 2.4 mg/kg of body weight, followed 12 h later by an i.v. injection at 1.2 mg/kg of body weight.
Inclusion criteria.
Patients aged 18 to 120 months (inclusive) with a diagnosis of severe malaria were eligible, provided that a relative gave informed consent for the child to participate in the study. Severe malaria was defined as malaria (presence of >2,000 asexual forms of P. falciparum/μl assessed on a blood film) with one or more of the following features: Blantyre coma score of ≤2, repeated witnessed convulsions (three or more observed convulsions), lactate concentration in whole blood or capillary blood of ≥5 mmol/liter, glucose concentration in whole blood or capillary blood of ≤2.2 mmol/liter, or severe anemia (hemoglobin concentration of <5 g/dl and/or hematocrit concentration of <15%).
Exclusion criteria.
Children with uncomplicated or moderate malaria as well as those with known sickle cell disease were excluded. Also excluded were patients who had taken ARS or quinine within 12 h of the clinical assessment. Alternative diagnoses were excluded clinically and by additional investigations (such as cerebrospinal fluid examination or chest radiography), as appropriate.
Screening of patients.
The admitting physicians referred children with a suspected diagnosis of malaria to the study team. All children with a positive blood film were then screened by taking of a history, performance of a physical examination, and measurement of lactate, glucose, and hematocrit concentrations in a heparinized blood sample (2 ml). Whole-blood lactate and glucose concentrations were measured with a YSI 2300 analyzer (YSI Instrument Co. Inc., Yellow Springs, Ohio); the packed cell volume (PCV) was estimated by using a microhematocrit centrifuge (Hawksley, Lancing, United Kingdom); thick and thin blood films were stained with Giemsa or Field's stain, respectively; and counts were obtained as described before (6, 13). The films were defined as negative if there were no asexual forms of P. falciparum in 100 high-power fields of a thick film.
Antimalarials.
Parenteral ARS (Guilin No. 2 Factory, Guangxi, People's Republic of China) was used for this study. Ampoules containing 60 mg of ARS were supplied with solvent ampoules containing sodium bicarbonate (5%, 1 ml). ARS was administered i.v. as a bolus (<5 s) immediately after dilution by using a 1-ml syringe. ARS was injected i.m. into the anterolateral aspect of one thigh. Unused diluted ARS was stored for assay. Parenteral ARS (1.2 mg/kg once daily, i.v.) was continued until the child could tolerate oral treatment, and ARS was given for a minimum of 3 days. The children then received a curative dose of sulfadoxine-pyrimethamine (Fansidar; Roche), as follows: children ages <4 years, one-half tablet; children ages 5 to 6 years, one tablet; children ages ≥7 years, one and a half tablets.
Sampling and storage protocols.
On admission a plasma sample (from 2 ml of whole blood) was obtained from each patient. After ARS administration, sampling (0.5 ml of whole blood) for ARS and dihydroartemisinin (DHA) assays was done at the following times: 1, 3, 6, 10, 15, 45, and 90 min and 2, 4, 8, and 12 h. When the crossover treatment was begun, an identical schedule was followed immediately after the last sample of the preceding schedule was obtained (12 h). Whole blood was collected and placed into prechilled, heparinized microcentrifuge tubes. Plasma was separated within 15 min of collection (10,000 × g, 5 min). Storage and transport of specimens was at −80°C.
Patient monitoring.
Patients were under the care of dedicated study physicians. After admission, vital signs (pulse, blood pressure, respiratory rate, auricular temperature) and coma scores were recorded at the same time that blood was sampled for pharmacokinetic analysis and then at 4- to 6-h intervals or more frequently if clinically indicated.
Concomitant therapy.
General supportive care and fluid management were as described previously (1). Fever was treated with acetaminophen suppository (Efferalgan pediatric; UPSA), and seizures were managed with i.v. diazepam (0.3 mg/kg; Valium; Roche), as indicated. Severe anemia (PCV < 15% or hemoglobin concentration < 5 g/dl) was corrected by transfusion of packed cells screened for infection (including human immunodeficiency virus). Anemic children were given ferrous fumarate syrup (2.5 to 5 ml daily; Fersamal; Forley, Harrow, United Kingdom) and folic acid (5 mg once daily; Cox Continental, Devon, United Kingdom) for 14 days at the time of discharge.
Follow-up.
Patients received follow-up examinations between 7 and 30 days after entry into the study. A full physical examination, including neurological assessment and screening laboratory tests, was carried out. Relatives were encouraged to return to the hospital earlier if the child developed a fever or other symptoms. Patients with P. falciparum parasitemia or other complications at follow-up were treated and asked to return for a further follow-up appointment.
Drug assay.
The assay of ARS and DHA in plasma samples was by a specific and selective high-pressure liquid chromatography (HPLC) method with electrochemical detection (ECD) operating in the reductive mode (1, 2, 10, 15). For ARS, the between-run coefficients of variation (CVs) were 9.98 and 3.67% for concentrations of 45 and 180 ng/ml, respectively. In addition, the CVs for day-to-day experiments for concentrations of 750 and 1,050 ng/ml were 1.13 and 5.80%, respectively. For DHA, the between-run CVs were 7.13% for 45 ng/ml and 3.27% for 180 ng/ml. For day-to-day experiments the CVs were 1.44 and 6.06% for concentrations of 750 and 1,050 ng/ml, respectively. The limits of quantification of HPLC-ECD (defined as the peak height at a signal-to-noise ratio of 3 at a sensitivity of −50 nA in a 1-ml plasma sample) in spiked plasma samples were 5 ng/ml for both compounds.
Pharmacokinetic analyses.
Pharmacokinetic modeling was carried out with WinNonLin software (version 3.0; Pharsight Corp., Mountain View, Calif.). Conversion of ARS to DHA was assumed to be complete. For both i.v. and i.m. analyses the Gauss-Newton minimization method was used. One- and two-compartment models were examined for goodness of fit by using standard criteria (including inspection and the Aikake information criterion). A two-compartment model was preferred for both the i.v. and the i.m. routes. Bioavailability estimates were based on AUCi.m./AUCi.v., where AUC is the area under the concentration-time curve.
Doses were converted to molar equivalents to allow comparability between ARS (molecular mass, 384) and DHA (molecular mass, 284) levels. The ARS remaining in the syringe after dosing was stored and assayed, and the amounts determined were used for pharmacokinetic analyses. The mean ± standard deviation (SD) ARS concentration was 63 ± 10.4 mg/ml, with a range from 19.5 to 83.6 mg/ml. This large range reflects one low outlying value.
Statistical analysis.
Data were analyzed by using SYSTAT (version 5.2; SYSTAT, Inc., Evanston, Ill.) or Stata (version 7; Stata Corporation, College Station, Tex.) software. Normally distributed data were compared by parametric tests (Student's t test or multiple analysis of variance), and nonnormally distributed data were compared by the Kruskall-Wallis test. Pearson's product moment coefficient was used for correlation analysis. Measures repeated over time were compared by using the multivariate linear general hypothesis.
RESULTS
Three hundred thirty-six children were screened for eligibility for entry into this study. Of these, 144 (43%) had asexual P. falciparum parasites on blood film examination and 39 (27%) met the criteria for severe malaria. Five patients were ineligible because parenteral quinine treatment had been commenced by an attending physician, four patients were ineligible because they had received oral ARS in the community, and two patients were not included because the parents were unable to or refused to provide consent. This left 28 patients who were randomized.
Withdrawals and exclusions from pharmacokinetic analysis.
Two patients (patients ARS 5 and ARS 11) were excluded because i.v. access could not be obtained within a prespecified time. Patient ARS 28 died before any samples were obtained. Samples from patients ARS 17 and ARS 18 were not analyzed because some samples were spoiled. Two patients (patients ARS 3 and ARS 15) had artemisinin profiles in plasma that could not be adequately modeled. Taking into account these exclusions, there were 10 subjects in group I (who received i.v. first) and 11 subjects in group II (who received i.m. first) who yielded useful pharmacokinetic data.
Admission characteristics of patients.
All patients fulfilled at least one criterion (and frequently more than one criterion) for severe malaria (11). There were two fatal cases (overall mortality = 7%), with one patient dying within 20 min of admission and the other patient (patient ARS 13) dying after 6 h. Patient ARS 13 had adequate levels of ARS (peak level = 464 ng/ml) and DHA (peak level = 304 ng/ml) in samples that were available for analysis. The admission clinical, laboratory, and parasitological characteristics of the patients in each treatment group are shown in Table 1. These variables were comparable for each treatment group.
TABLE 1.
Admission variables for the two treatment groupsa
| Variable | Group I (n = 14) | Group II (n = 14) |
|---|---|---|
| Demographic | ||
| Age (mo) | 36 (23-48) | 21 (19-27) |
| No. of males:no. of females | 8/6 | 4/10 |
| Ht (cm) | 93 (83-100) | 82 (81-91) |
| Wt (kg) | 12.5 (11-14.5) | 12.3 (10.7-11.9) |
| Clinical | ||
| Temp (°C) | 38.5 (37.6-39.3) | 37.7 (36.6-39.4) |
| Pulse (no. of beats/min) | 160 (150-180) | 160 (130-170) |
| % Patients with Blantyre coma score ≤2 | 36 | 50 |
| Respiration (no. of breaths/min) | 55 (17) | 53 (13) |
| Laboratory | ||
| Hematocrit (%) | 15 (13-20) | 15 (12-29) |
| Geometric mean (95% CI) parasitemia/μl | 37,106 (8,422-163,483) | 42,405 (14,721-122,154) |
| Lactate concn (mmol/liter) | 8.5 (4.2) | 6.9 (4.4) |
| Glucose concn (mmol/liter) | 4.8 (3.8-5.2) | 5.1 (1.6-6.1) |
All values except lactate concentrations (which are means [SDs]) and those indicated otherwise are medians (interquartile ranges). CI, confidence interval.
Response to ARS treatment.
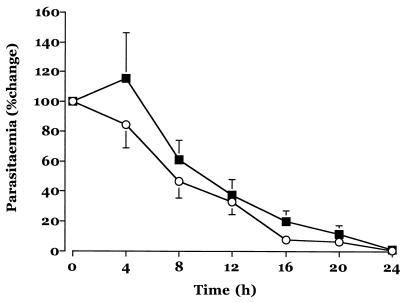
Fig. 1 shows the mean change in the level of parasitemia from the baseline level in the two treatment groups in the first 24 h after admission, and Table 2 summarizes the pharmacodynamic response parameters. Measures of parasite clearance were comparable between group I and group II, particularly in the first 12 h after admission, when the effects of only one route of administration are relevant. The median fall in the level of parasitemia from the baseline level was ∼70%. Lactate concentrations had normalized in most patients by 12 h after admission. There were no adverse events attributable to study drug.
FIG. 1.
Mean ± standard error of the mean change in parasitemia levels after admission for patients in group I (squares) and group II (circles).
TABLE 2.
Outcome variables for the two treatment groupsa
| Variable | Group I (n = 14) | Group II (n = 14) |
|---|---|---|
| Parasite | ||
| PC50 (h) | 8 (5-12) | 7 (6-11) |
| PC90 (h) | 16 (11-21) | 16 (10-19) |
| PCT (h) | 23 (12-24) | 18 (8-20) |
| PC12 (%) | 24 (7-51) | 32 (0-56) |
| Clinical | ||
| FCT (h) | 22 (12-48) | 14 (2-31) |
| Hospital stay (days) | 3 (2-3) | 2 (1-3) |
| Mortality (no. of patients treated/no. of patients who died) | 0/14 | 2/14 |
| Positive blood film day 28 | 1/9 | 0/9 |
| Laboratory | ||
| Lactate concn (mmol/liter) at 4 h | 2 (1.7-3.4) | 2.6 (1.4-3.6) |
| Lactate concn (mmol/liter) at 12 h | 0.9 (0.8-1.6) | 1.6 (1.2-2.5) |
All values except temperature which are means [SDs] and those indicated otherwise are medians (interquartile ranges). PC12, percent fall in parasitemia at 12 h; PC50, time for parasitemia to fall to 50% of the baseline value; PC90, time for parasitemia to fall 90% of the baseline value; PCT, parasite clearance time; FCT, fever clearance time. Group I received 2.4 mg of ARS/kg i.v. followed by 1.2 mg of ARS/kg i.m.; group II received 2.4 mg of ARS/kg i.m. followed by 1.2 mg/kg i.v.
Pharmacokinetic analyses. (i) i.v. ARS.
An open two-compartment model with no lag time and first-order elimination kinetics best fit the data. Modeling was excellent, with a mean ± SD correlation coefficient of 0.999 ± 0.003 and a median value for the Aikake information criterion of −13.4 (range, −108.3 to 29.8). The pharmacokinetic data are summarized in Table 3 .
TABLE 3.
Pharmacokinetics of ARS and DHA in children with severe malariaa
| Drug, route, and group | Actual dose (μmol/kg [mean ± SD]) | Vc/f (liters/kg) | Vc (liter/kg) | Vss/f (liter/kg) | Vss (liter/kg) | CL/f (liter/kg/min) | CL (liter/kg/min) |
|---|---|---|---|---|---|---|---|
| ARS | |||||||
| i.v. | |||||||
| Group I (n = 10) | 5.82 ± 1.72 | NA | 0.10 (0.03-0.6) | NA | 0.17 (0.09-4.1) | NA | 0.052 (0.002-0.17) |
| Group II (n = 11) | 3.31 ± 0.32 | NA | 0.21 (0.4-0.4) | NA | 0.44 (0.06-7.7) | NA | 0.071 (0.001-0.14) |
| Combined | NA | NA | 0.14 (0.03-0.6) | NA | 0.40 (0.06-7.7) | NA | 0.046 (0.001-0.17) |
| i.m. | |||||||
| Group I | 3.38 ± 0.33 | 1.3 (0.5-3.2) | 2.31 (0.2-9.3) | 2.07 (0.9-5.1) | 2.66 (0.2-11.8) | 0.040 (0.005-0.34) | 0.042 (0.001-0.93) |
| Group II | 6.95 ± 0.74 | 2.1 (0.3-6.4) | 1.37 (0.7-20.2) | 3.98 (0.3-9.1) | 2.16 (0.7-28.6) | 0.058 (0.003-0.17) | 0.045 (0.006-0.53) |
| Combined | NA | 1.9 (0.3-6.4) | 1.80 (0.2-20.2) | 2.67 (0.3-9.1) | 2.55 (0.2-28.6) | 0.046 (0.003-0.34) | 0.043 (0.001-0.93) |
| DHA | |||||||
| i.v. | |||||||
| Group I | 5.82 ± 1.72 | NA | 0.47 (0.05-1.2) | NA | 0.75 (0.3-1.3) | NA | 0.036 (0.012-0.15) |
| Group II | 3.31 ± 0.32 | NA | 0.47 (0.3-1.5) | NA | 0.77 (0.5-1.9) | NA | 0.018 (0.011-0.04) |
| Combined | NA | NA | 0.47 (0.05-1.5) | 0.77 (0.3-1.9) | NA | 0.025 (0.011-0.15) | |
| i.m. | |||||||
| Group I | 3.38 ± 0.33 | 1.2 (0.4-6.3) | 1.44 (0.1-55.1) | 1.32 (0.5-7.9) | 1.32 (0.5-7.9) | 0.036 (0.001-0.18) | 0.037 (0.02-1.58) |
| Group II | 6.95 ± 0.74 | 1.2 (0.03-3.2) | 1.10 (0.1-2.4) | 1.28 (0.5-4.2) | 1.28 (0.5-4.2) | 0.025 (0.006-0.13) | 0.0201 (0.001-0.096) |
| Combined | NA | 1.2 (0.03-6.3) | 1.36 (0.1-55.1) | 1.32 (0.5-7.9) | 1.30 (0.5-7.9) | 0.025 (0.006-0.18) | 0.0280 (0.001-1.58) |
Abbreviations: Vc/f, fractional volume of the central compartment; Vc, volume of the central compartment; Vss, volume of distribution at steady state; CL/f, fractional clearance; C L, total body clearance of the drug from plasma; t1/2α, distribution half-life; t1/2β, elimination half life; tabs, half-life of appearance; AUC, area under the plasma concentration-time curve; Tmax, time to reach maximum concentration following drug administration; Cmax, peak drug concentration in plasma after administration of a single dose.
The median AUC and the maximum concentration of drug in plasma after administration of a single dose (Cmax) for ARS were approximately twice as large in patients who received the loading dose of ARS (2.4 mg/kg, group I) compared with the values in those who received a maintenance dose 12 h after admission (1.2 mg/kg, group II). The differences in Cmaxs were significant (P = 0.004), but the differences in AUCs were not significant (P > 0.1). The clearance of i.v. ARS was comparable between the two study groups. Large interindividual variances in estimates for AUC and elimination half-lives may reflect sparse data for ARS during the elimination phase because of its very short half-life. The volumes of distribution of the central compartment (Vc) and at steady state (Vss) were higher (by about twofold) in patients who had already received a dose of ARS (i.e., group II versus group I; P = 0.033 for comparison of Vc values, but P > 0.3 for comparison of Vss values), but the large variabilities in the estimates for these variables do not suggest that these are clinically important differences.
(ii) i.m. ARS.
An open two-compartment model with no lag time, first-order absorption, and first-order elimination kinetics best fit the data. Modeling was very good, with a mean ± SD correlation coefficient of 0.91 ± 0.12 and a median value for the Aikake information criterion of 3.4 (range, −37.0 to 66.2). The pharmacokinetic data are summarized in Table 3. As for the pharmacokinetic behavior of i.v. ARS, there were no significant differences in AUC and elimination half-life estimates between group I and group II or between the i.v. and the i.m. route. Cmax values were also not significantly different between the two groups (P > 0.4). Bioavailability estimations for i.m. ARS were similar for both group I and group II, with a combined median value of 66% (range, 24 to 742%). Partly because of the very rapid conversion of ARS to DHA, the large variabilities in the estimates of the AUCs for i.v. ARS are probably sufficient to explain the observed variability in the relative bioavailability of i.m. ARS.
(iii) DHA kinetics after i.v. ARS.
An open two-compartment model with no lag time, first-order appearance, and first-order elimination kinetics best fit the data. Modeling was excellent, with a mean ± SD correlation coefficient of 0.99 ± 0.19 and a median value for the Aikake information criterion of 4.7 (range, −29.0 to 37). As expected, Cmaxs were twice as high for group I patients than for group II patients (P = 0.013), although variability in the estimates of AUC did not result in significant differences between the two groups (P = 0.2). The distribution half-life was significantly shorter for group I patients than for group II patients (median values, 0.31 and 7.1 min, respectively; P = 0.04). There were no other significant differences in pharmacokinetic parameters between the two groups, and the variabilities of these estimates were much smaller than those for the corresponding estimates for kinetic parameters for ARS. This probably reflects better modeling because of the longer elimination half-lives of DHA.
DHA kinetics after i.m. ARS.
An open two-compartment model with no lag time, first-order appearance, and first-order elimination kinetics best fit the data. Modeling was excellent, with a mean ± SD correlation coefficient of 0.96 ± 0.06 and a median value for the Aikake information criterion of −14.8 (range, −45 to 19). Both Cmax and AUC estimates were significantly higher (by approximately twofold) for group II patients than for group I patients (P = 0.03 and P = 0.02, respectively). There were no differences in any other estimates of the pharmacokinetic parameters between the two groups. The overall estimate of the relative bioavailability of DHA after i.m. ARS was a median of 86% (range, 11 to 462%). Again, this large range reflects large (10-fold) variations in estimates for the AUC for DHA after both i.v. and i.m. ARS.
Pharmacokinetic-pharmacodynamic relations.
There was no relationship between the pharmacokinetic parameters obtained in the first 12 h of the study and estimates of parasite clearance at 12 h (reflecting the effects of a single dose), even after the AUCs for ARS and DHA were combined for individual patients.
Adverse events.
There were no major adverse events attributable to ARS. The i.m. ARS injection was not painful, and there was no other local toxicity. However, there were two complicated cases that are described in greater detail below.
(i) Patient ARS 4.
Patient ARS 4 was a 56-month-old boy who presented with a history of fever, convulsions, and vomiting. He had vomited one dose of oral Quinimax given at the time of admission to the hospital. On examination he was pale and conscious (Blantyre coma score = 5/5), and he had splenomegaly (5 cm) and hepatomegaly (2 cm). His lactate and glucose concentrations were 2.9 and 5.7 mmol/liter, respectively, and he had a PCV of 13%, a hemoglobin concentration of 3.4 g/dl, a white cell count of 3.4 × 109/liter (41% lymphocytes), a platelet count of 134 × 109/liter, and a parasitemia level of 2,738/μl. He developed macroscopic hemoglobinuria within 1 h of admission and randomization to group I and became jaundiced. He received a blood transfusion (270 ml of packed cells). His hemoglobin value stabilized at >8 g/dl, and his urine cleared over 2 days. He made an otherwise uncomplicated recovery.
(ii) Patient ARS 14.
Patient ARS 14 was a 49-month-old boy who presented with a history of fever without convulsions or coma. He had received traditional treatment prior to admission. He was pale and jaundiced but without hepatosplenomegaly. His admission lactate and glucose concentrations were 6.1 and 5.1 mmol/liter, respectively, with a PCV of 13%, a hemoglobin concentration of 4.3 g/dl, and a parasitemia level of 15,825/μl; he was randomized to group I. He passed black urine in diminishing volumes overnight (<50 ml in the day following admission). This oliguria lasted for 48 h but was associated with elevations in creatinine and urea concentrations (peak values, 342 μmol/liter and 34.8 mmol/liter, respectively, 48 h after admission) that were sustained for 7 days after admission. He was referred for specialized nephrological care but did not attend any follow-up appointments.
DISCUSSION
Parenteral dosing is the preferred route for treatment of patients with severe malaria. i.v. administration of antimalarials eliminates the risk of poor or erratic bioavailability that may arise when other routes of administration are used. However, the i.m. route is often more convenient than the i.v. route, particularly when venous access is difficult to establish, as is frequently the case for severely ill young children. Water-soluble artemisinin derivatives such as ARS allow formal bioavailability assessments because formulations suitable for the i.v. route of administration can be compared directly with those suitable for the i.m. route of administration. This is in contrast to the situation for artemether, an oil-soluble artemisinin derivative, which must be given by the i.m. route and whose bioavailability has not been established in severe malaria because an i.v. formulation is lacking.
Our study estimates that the absolute bioavailability of i.m. ARS is ∼90% (for the main antimalarial metabolite, DHA), although this value is encompassed by a large range (11 to 462%). This variability is probably due to the disposition of ARS and DHA rather than other factors, because similar variations in AUCs are observed for DHA when ARS is given by the i.v. route (5- to 10-fold) compared with those obtained when ARS is given by the i.m. route (5- to 15-fold; Table 3). Absorption is rapid, with Cmax being achieved in less than 1 h in the majority of children. No pharmacodynamic differences (assessed by parasite clearance estimates) between the i.m. and i.v. routes were seen (Table 2), suggesting that even the lowest AUCs did not compromise parasiticidal activity. This is unsurprising, as the 50% inhibitory concentrations (IC50s) from in vitro assays defining the IC50s of ARS are far below those achieved in vivo (14). The variabilities in other pharmacokinetic parameters observed for ARS and DHA after i.m. and i.v. administration of ARS are also large. However, clearance of DHA is much less variable when ARS is given by the i.v. route than by the i.m. route (in a comparison of the ranges of these estimates between groups I and II, the i.v. route has 9-fold variation; in contrast, the i.m. route has 100-fold variation). The longer estimates for terminal elimination half-lives for DHA given by the i.m. route suggest that in some patients absorption from the i.m. site may be rate limiting for elimination (flip-flop kinetics).
The rapid elimination of ARS suggests that this parent compound does not have significant in vivo antimalarial activity in most patients. Most discussion of bioavailability is therefore focused on DHA, with data for ARS being included for completeness. The consistent pharmacodynamic response to the regimen of parenteral ARS used in this study confirms that such regimens are appropriate for use in patients with severe malaria. Furthermore, the parenteral ARS given to children with severe malaria in this study was not associated with any attributable systemic or local toxicity, was easy to administer, and rapidly became the preferred antimalarial at the study site. Unlike quinine, ARS is not associated with hyperinsulinemia and therefore does not increase the risk of hypoglycemia beyond that associated with underlying disease (1).
The pharmacokinetic parameters for DHA (after i.v. ARS administration) in this study are comparable to those obtained in Ghanaian children with moderate malaria who received an identical formulation of i.v. ARS (6). This comparison (for example, of Cmax, AUC, clearance, and Vss values) suggests (even though the comparison is between the previous study, which used a one-compartment analysis, and the present study, which used a two-compartment analysis) that, unlike quinine, the severity of disease does not have an important influence on the pharmacokinetic behavior of ARS (8). These findings are also consistent with observations for Vietnamese adults, in whom disease severity did not influence the pharmacokinetic behavior of i.v. ARS (3). Larger studies will be needed to understand the mechanisms that influence variability in the disposition of ARS, but our analysis suggests that host factors are more important than disease severity.
When a child presents with severe malaria to a hospital in sub-Saharan Africa, many factors influence the eventual outcome. These include both the rapidity of diagnosis and the speed with which effective treatment is given, especially as most deaths occur within the first 12 h after admission. At present there is no alternative therapy to quinine given in a loading dose by either the i.m. route (5) or the i.v. route. However, our study shows that i.m. ARS is an important alternative to quinine. In children with severe malaria, a much larger study comparing quinine with ARS is needed to determine if pharmacodynamic differences between ARS and quinine can translate to improvements in terms of reductions in rates of mortality.
TABLE 3.
—Continued
| t1/2α (min) | t1/2β (min) | tabs (min) | AUC (μmol · min/liter) | Relative bioavailability (%) | Tmax (min) | Cmax (μmol/liter) |
|---|---|---|---|---|---|---|
| 2.1 (0.9-5.4) | 1.5 (0.06-1668) | NA | 162.7 (49.9-5071.4) | NA | NA | 77.2 (13.5-266.3) |
| 1.4 (0.5-2.1) | 11.5 (0.68-2023) | NA | 86.7 (47.2-2660.4) | NA | NA | 38.98 (16.4-49.8) |
| 1.6 (0.5-5.4) | 2.9 (0.06-2023) | NA | NA | NA | NA | NA |
| 7.7 (2.3-17.6) | 25.2 (4.23-501.00) | 2.7 (0.87-5.99) | 83.5 (9.2-747.3) | 54.1 (24.4-741.8) | 7.2 (4.1-11.4) | 1.6 (0.6-2.97) |
| 5.2 (1.4-52.8) | 48.2 (13.37-319.65) | 2.5 (1.28-41.52) | 84.9 (39.2-2264.7) | 94.4 (31.3-250.2) | 8.0 (3.9-78.9) | 1.72 (0.7-10.1) |
| 5.96 (1.4-52.8) | 42.3 (4.23-501.00) | 2.7 (0.87-41.52) | NA | 65.7 (24.4-741.8) | 7.2 (3.9-78.9) | NA |
| 0.3 (0.1-28.0) | 20.7 (0.28-35.81) | 0.2 (0.05-4.96) | 194.8 (52.4-498.8) | NA | 0.5 (0.1-14.3) | 10.59 (5.0-75.4) |
| 7.2 (0.5-54.4) | 32.0 (14.11-53.55) | 0.4 (0.05-2.17) | 155.6 (84.2-334.2) | NA | 1.4 (0.3-7.3) | 5.57 (1.8-8.5) |
| 0.8 (0.1-54.4) | 22.8 (0.28-52.55) | 0.2 (0.05-4.96) | NA | NA | 1.3 (0.1-14.3) | NA |
| 17.6 (2.4-36.6) | 31.9 (18.2-110.4) | 15.4 (3.8-47.9) | 83.6 (17.4-298.2) | 84.7 (11.4-462.1) | 25.9 (10.8-71.9) | 1.2 (0.1-2.9) |
| 8.7 (3.2-21.6) | 40.2 (1.4-148.8) | 25.1 (5.1-47.3) | 236.9 (46.7-582.7) | 109.4 (36.1-408.3) | 40.5 (11.5-68.2) | 2.2 (0.8-3.6) |
| 10.8 (2.4-36.6) | 35.8 (1.4-148.8) | 18.4 (3.8-47.9) | NA | 86.37 (11.4-462.1) | 35.1 (10.8-71.9) | NA |
Acknowledgments
We thank the medical and nursing staff of the emergency room and general pediatric ward of the Centre Hospitalier de Libreville, especially M. Josseaume, N. Engohan, M. Tchoua, and R. Tchoua for advice and support in conducting this study. We also thank Nze Pascal Christian, N. Obiang Nestor, Ekoumembia Michel, and Mbadinga Frankie for assistance.
C. Nealon was supported by a grant from the Special Trustees of St. George's Hospital Medical School. This work was funded by the World Health Organization and forms part of a program of research into clinical tropical medicine funded by the Wellcome Trust.
The first three authors contributed equally to the work.
REFERENCES
- 1.Agbenyega, T., B. Angus, G. Bedu-Addo, B. Baffoe-Bonnie, T. Guyton, P. Stacpoole, and S. Krishna. 2000. Glucose and lactate kinetics in children with severe malaria. J. Clin. Endocrinol. Metab. 85:1569-1576. [DOI] [PubMed] [Google Scholar]
- 2.Barradell, L. B., and A. Fitton. 1995. Artesunate. A review of its pharmacology and therpeuctic efficacy in the treatment of malaria. Drugs 50:714-741. [DOI] [PubMed] [Google Scholar]
- 3.Davis, T., H. Phuong, K. Ilett, N. Hung, K. Batty, V. Phuong, S. Powell, H. Thien, and T. Binh. 2001. Pharmacokinetics and pharmacodynamics of intravenous artesunate in severe falciparum malaria. Antimicrob. Agents Chemother. 45:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hien, T. T., N. P. J. Day, N. H. Phu, N. T. H. Mai, T. T. H. Chau, P. P. Loc, D. X. Sinh, L. V. Chuong, H. Vinh, D. Waller, T. E. A. Peto, and N. J. White. 1996. A controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N. Engl. J. Med. 335:76-83. [DOI] [PubMed] [Google Scholar]
- 5.Krishna, S., N. V. Nagaraja, T. Planche, T. Agbenyega, G. Bedo-Addo, D. Ansong, A. Owusu-Ofori, A. L. Shroads, G. Henderson, A. Hutson, H. Derendorf, and P. W. Stacpoole. 2001. Population pharmacokinetics of intramuscular quinine in children with severe malaria. Antimicrob. Agents Chemother. 45:1803-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishna, S., T. Planche, T. Agbenyega, C. Woodrow, D. Agranoff, G. Bedu-Addo, A. K. Owusu-Ofori, J. A. Appiah, S. Ramanathan, S. M. Mansor, and V. Navaratnam. 2001. Bioavailability and preliminary clinical efficacy of intrarectal artesunate in Ghanaian children with moderate malaria. Antimicrob. Agents Chemother. 45:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishna, S., D. Waller, F. ter Kuile, D. Kwiatkowski, J. Crawley, C. F. C. Craddock, F. Nosten, D. Chapman, D. Brewster, P. A. Holloway, and N. J. White. 1994. Lactic acidosis and hypoglycaemia in children with severe malaria: pathophysiological and prognostic significance. Trans. R. Soc. Trop. Med. Hyg. 88:67-73. [DOI] [PubMed] [Google Scholar]
- 8.Krishna, S., and N. J. White. 1996. Pharmacokinetics of quinine, chloroquine and amodiaquine. Clinical implications. Clin. Pharmacokinet. 30:263-299. [DOI] [PubMed] [Google Scholar]
- 9.Murphy, S., M. English, C. Waruiru, I. Mwangi, E. Amukoye, J. Crawley, C. Newton, P. Winstanley, N. Peshu, and K. Marsh. 1996. An open randomized trial of artemether versus quinine in the treatment of cerebral malaria in African children. Trans. R. Soc. Trop. Med. Hyg. 90:298-301. [DOI] [PubMed] [Google Scholar]
- 10.Navaratnam, V., M. N. Mordi, and S. M. Mansor. 1997. Simultaneous determination of artesunate and dihydroartemisinin in blood plasma by high-performance liquid chromatography for application in clinical pharmacological studies. J. Chromatogr. 692:157-162. [DOI] [PubMed] [Google Scholar]
- 11.Newton, C. W., and S. Krishna. 1998. Severe falciparum malaria in children: current understanding of its pathophysiology and supportive treatment. Pharmacol. Ther. 79:1-53. [DOI] [PubMed] [Google Scholar]
- 12.Newton, P., Y. Suputtamongkol, P. Teja-Isavadharm, S. Pukrittayakamee, V. Navaratnam, I. Bates, and N. White. 2000. Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob. Agents Chemother. 44:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Planche, T., S. Krishna, M. Kombila, K. Engel, J. Faucher, E. Ngou-Milama, and P. Kremsner. 2001. A comparison of laboratory methods for the assessment of children with malaria. Am. J. Trop. Med. Hyg. 65:599-602. [DOI] [PubMed] [Google Scholar]
- 14.Price, R., C. A. Cassar, A. Brockman, M. Duraisingh, M. van Vugt, N. J. White, F. Nosten, and S. Krishna. 1999. Pfmdr1 gene amplification and multidrug resistant Plasmodium falciparum on the northwest border of Thailand. Antimicrob. Agents Chemother. 43:2943-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Agtmael, M., J. Butter, E. Portier, and C. van Boxtel. 1998. Validation of an improved reversed-phase high-performance liquid chromatography assay with reductive electrochemical detection for the determination of artemisinin derivatives in man. Ther. Drug Monit. 20:109-116. [DOI] [PubMed] [Google Scholar]
- 16.van Hensbroek, M. B., E. Onyiorah, S. Jaffer, G. Schneider, A. Palmer, J. Frenkel, G. Enwere, S. Forck, A. Nusmeijer, S. Bennett, B. Greenwood, and D. Kwiatkowski. 1996. A trial of artemether or quinine in children with cerebral malaria. N. Engl. J. Med. 335:69-75. [DOI] [PubMed] [Google Scholar]
- 17.Winstanley, P. A., S. A. Ward, and R. W. Snow. 2002. Clinical status and implications of antimalarial drug resistance. Microbes Infect. 4:157-164. [DOI] [PubMed] [Google Scholar]