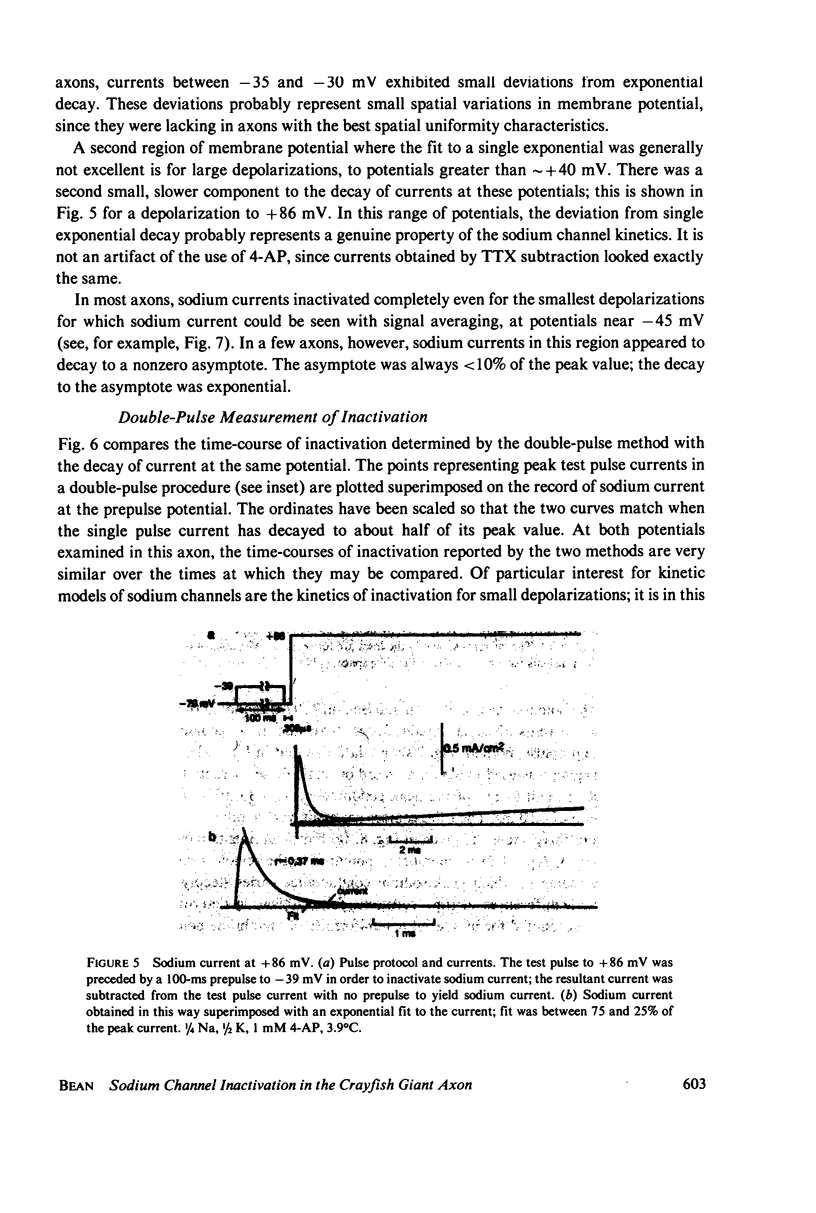
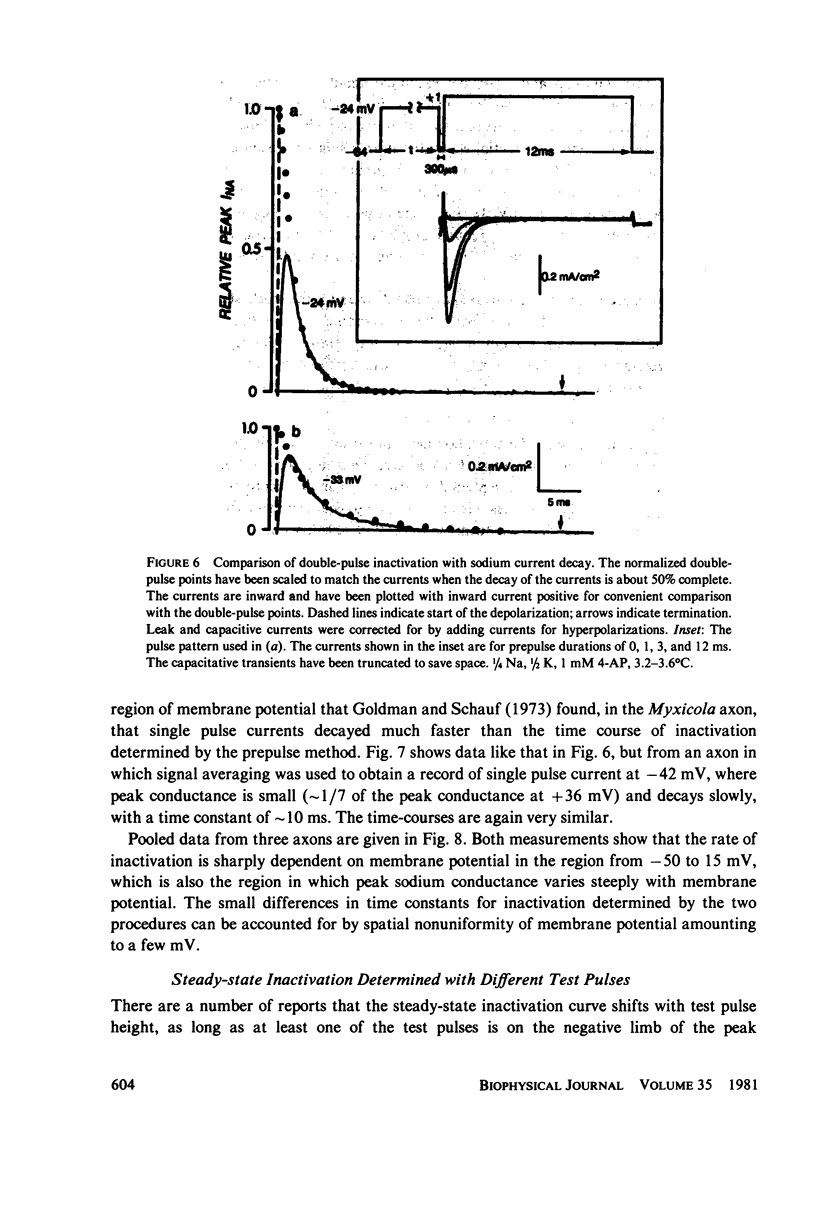
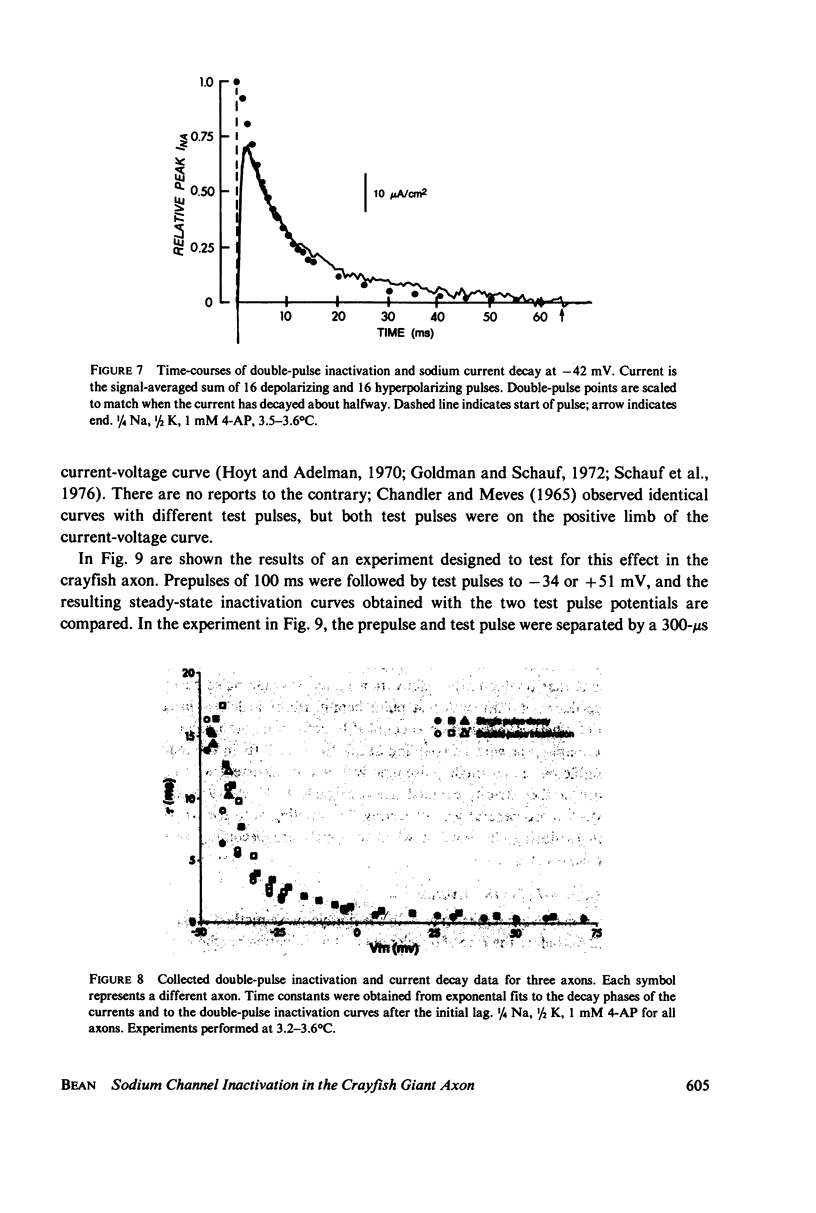
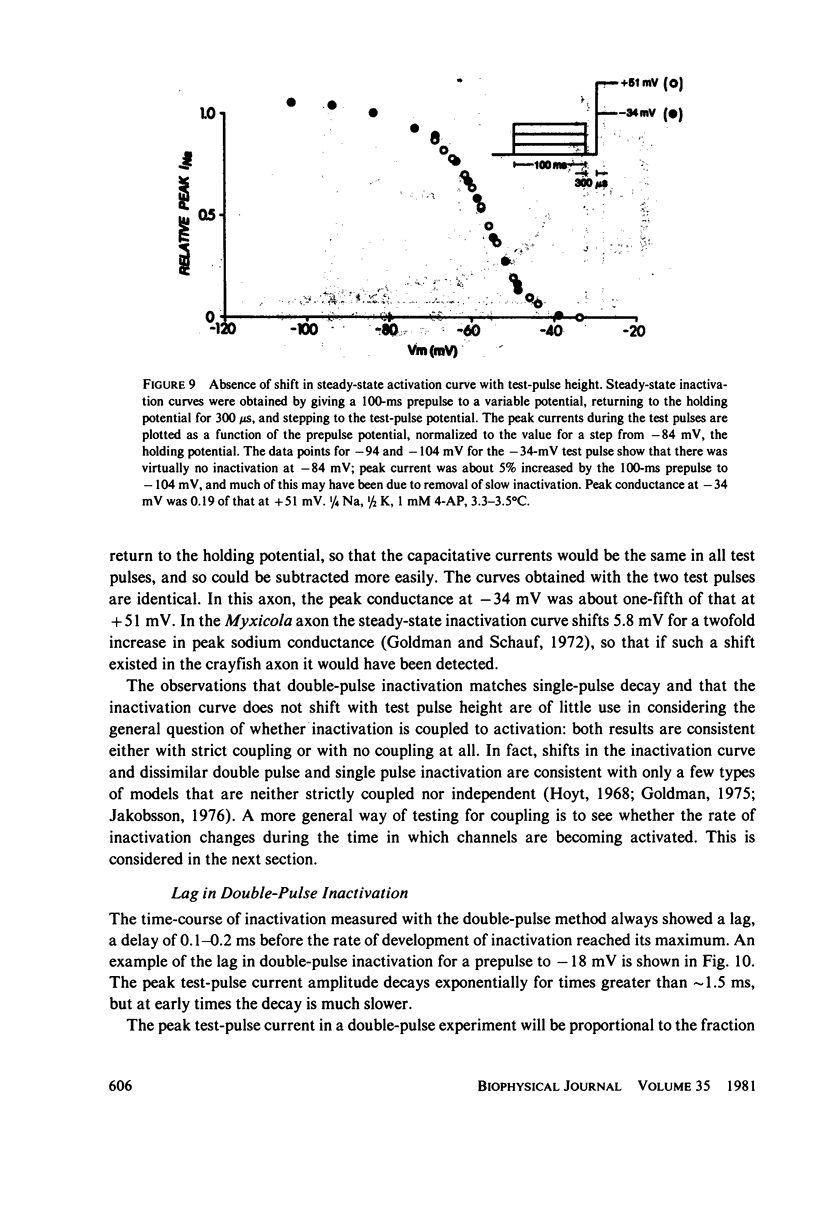
Abstract
Experiments on sodium channel inactivation kinetics were performed on voltage-clamped crayfish giant axons. The primary goal was to investigate whether channels must open before inactivating. Voltage-clamp artifacts were minimized by the use of low-sodium solutions and full series resistance compensation, and the spatial uniformity of the currents was checked with a closely spaced pair of electrodes used to measure local current densities. For membrane potentials between -40 and +40 mV, sodium currents decay to zero with a single exponential time-course. The time constant for decay is a steep function of membrane potential. The time-course of inactivation measured with the double-pulse method is very similar to the decay of current at the same potential. Steady-state inactivation curves measured with different test pulses are identical. The time-course of double pulse inactivation shows a lag that roughly correlates with the opening of sodium channels, but detailed comparisons with the time course of the prepulse current suggest that it is not strictly necessary for channels to open before inactivating. Measurements of the potential dependence of the integral of sodium conductance area also inconsistent with the simplest cases of models in which channels must open before inactivating.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Gilly W. F. Fast and slow steps in the activation of sodium channels. J Gen Physiol. 1979 Dec;74(6):691–711. doi: 10.1085/jgp.74.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock J. O., Schauf C. L. Combined voltage-clamp and dialysis of Myxicola axons: behaviour of membrane asymmetry currents. J Physiol. 1978 May;278:309–324. doi: 10.1113/jphysiol.1978.sp012306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Ionic current measurements in the squid giant axon membrane. J Gen Physiol. 1960 Sep;44:123–167. doi: 10.1085/jgp.44.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Almers W. Block of sodium conductance and gating current in squid giant axons poisoned with quaternary strychnine. Biophys J. 1979 Jul;27(1):57–73. doi: 10.1016/S0006-3495(79)85202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. C., Brodwick M. S., Oxford G. S., Rudy B. Arginine-specific reagents remove sodium channel inactivation. Nature. 1978 Feb 2;271(5644):473–476. doi: 10.1038/271473a0. [DOI] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The time course of sodium inactivation in squid giant axons. J Physiol. 1980 Feb;299:289–307. doi: 10.1113/jphysiol.1980.sp013125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L. Kinetics of channel gating in excitable membranes. Q Rev Biophys. 1976 Nov;9(4):491–526. doi: 10.1017/s0033583500002651. [DOI] [PubMed] [Google Scholar]
- Goldman L. Quantitative description of the sodium conductance of the giant axon of Myxicola in terms of a generalized second-order variable. Biophys J. 1975 Feb;15(2 Pt 1):119–136. doi: 10.1016/s0006-3495(75)85796-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Inactivation of the sodium current in Myxicola giant axons. Evidence for coupling to the activation process. J Gen Physiol. 1972 Jun;59(6):659–675. doi: 10.1085/jgp.59.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Quantitative description of sodium and potassium currents and computed action potentials in Myxicola giant axons. J Gen Physiol. 1973 Mar;61(3):361–384. doi: 10.1085/jgp.61.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt R. C., Adelman W. J., Jr Sodium inactivation. Experimental test of two models. Biophys J. 1970 Jul;10(7):610–617. doi: 10.1016/S0006-3495(70)86323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt R. C. Sodium inactivation in nerve fibers. Biophys J. 1968 Oct;8(10):1074–1097. doi: 10.1016/S0006-3495(68)86540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson E. An assessment of a coupled three-state kinetic model for sodium conductance changes. Biophys J. 1976 Apr;16(4):291–301. doi: 10.1016/S0006-3495(76)85689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. Inactivation of the sodium permeability in squid giant nerve fibres. Prog Biophys Mol Biol. 1978;33(2):207–230. doi: 10.1016/0079-6107(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Meves H., Vogel W. Inactivation of the asymmetrical displacement current in giant axons of Loligo forbesi. J Physiol. 1977 May;267(2):377–393. doi: 10.1113/jphysiol.1977.sp011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonner W. Relations between the inactivation of sodium channels and the immobilization of gating charge in frog myelinated nerve. J Physiol. 1980 Feb;299:573–603. doi: 10.1113/jphysiol.1980.sp013143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S., Pooler J. P. Selective modification of sodium channel gating in lobster axons by 2, 4, 6-trinitrophenol: Evidence for two inactivation mechanisms. J Gen Physiol. 1975 Dec;66(6):765–779. doi: 10.1085/jgp.66.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peganov E. M. O kinetike protsessa inaktivatsii natrievykh kanalov v perekhvate Ranv'e liagushki. Biull Eksp Biol Med. 1973 Nov;76(11):5–9. [PubMed] [Google Scholar]
- Provencher S. W. A Fourier method for the analysis of exponential decay curves. Biophys J. 1976 Jan;16(1):27–41. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Davis F. A. Further studies of activation-inactivation coupling in Myxicola axons. Insensitivity to changes in calcium concentration. Biophys J. 1975 Nov;15(11):1111–1116. doi: 10.1016/S0006-3495(75)85887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Pencek T. L., Davis F. A. Activation-inactivation coupling in Myxicola giant axons injected with tetraethylammonium. Biophys J. 1976 Sep;16(9):985–989. doi: 10.1016/S0006-3495(76)85749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoukimas J. J. Effect of calcium upon sodium inactivation in the giant axon of Loligo pealei. J Membr Biol. 1978 Jan 18;38(3):271–289. doi: 10.1007/BF01871926. [DOI] [PubMed] [Google Scholar]
- Shoukimas J. J., French R. J. Incomplete inactivation of sodium currents in nonperfused squid axon. Biophys J. 1980 Nov;32(2):857–862. doi: 10.1016/S0006-3495(80)85021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P. Ionic conductance changes in voltage clamped crayfish axons at low pH. J Gen Physiol. 1974 Dec;64(6):666–690. doi: 10.1085/jgp.64.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Jr Gating charge immobilization and sodium current inactivation in internally perfused crayfish axons. Nature. 1980 Oct 16;287(5783):644–645. doi: 10.1038/287644a0. [DOI] [PubMed] [Google Scholar]
- TAYLOR R. E., MOORE J. W., COLE K. S. Analysis of certain errors in squid axon voltage clamp measurements. Biophys J. 1960 Nov;1:161–202. doi: 10.1016/s0006-3495(60)86882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Armstrong C. M. Immobilisation of gating charge by a substance that simulates inactivation. Nature. 1978 Jun 1;273(5661):387–389. doi: 10.1038/273387a0. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Narahashi T. Kinetic analysis of pancuronium interaction with sodium channels in squid axon membranes. J Gen Physiol. 1977 Mar;69(3):293–323. doi: 10.1085/jgp.69.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Interactions of aminopyridines with potassium channels of squid axon membranes. Biophys J. 1976 Jan;16(1):77–81. doi: 10.1016/S0006-3495(76)85663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


