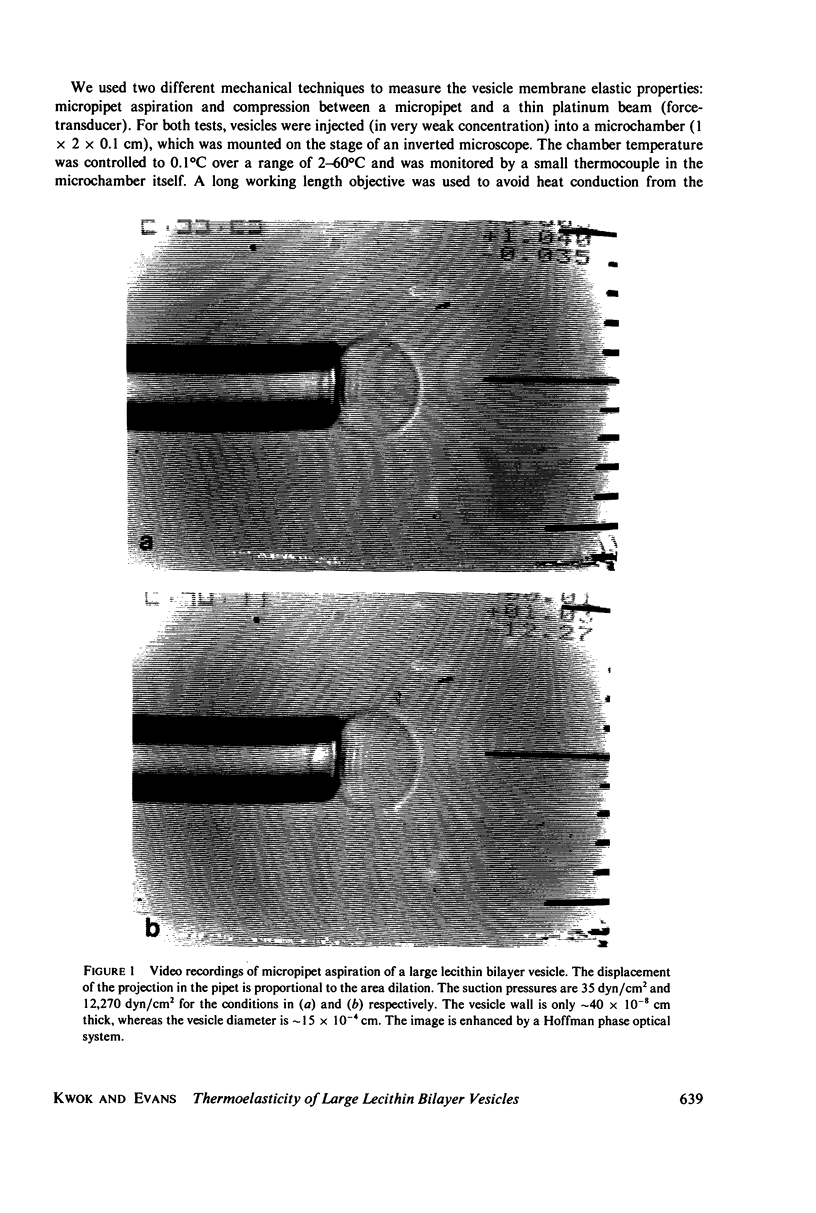
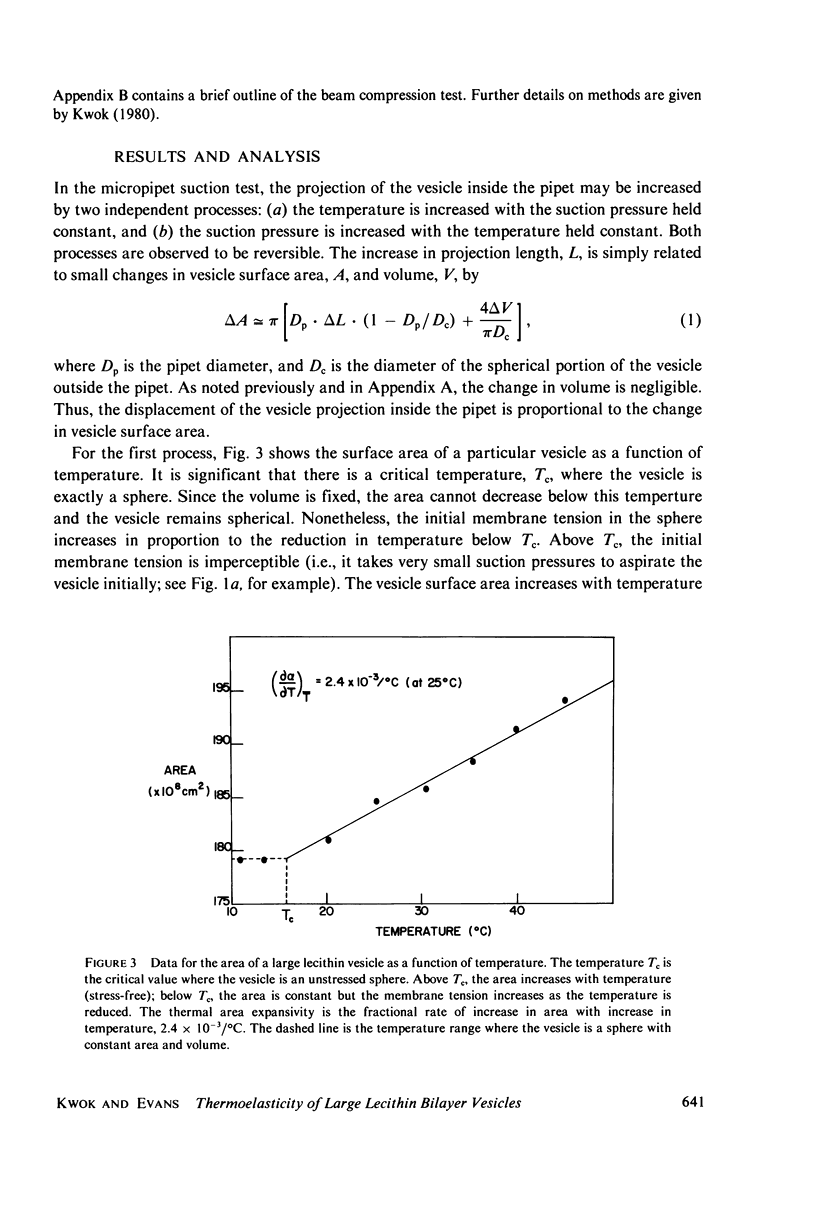
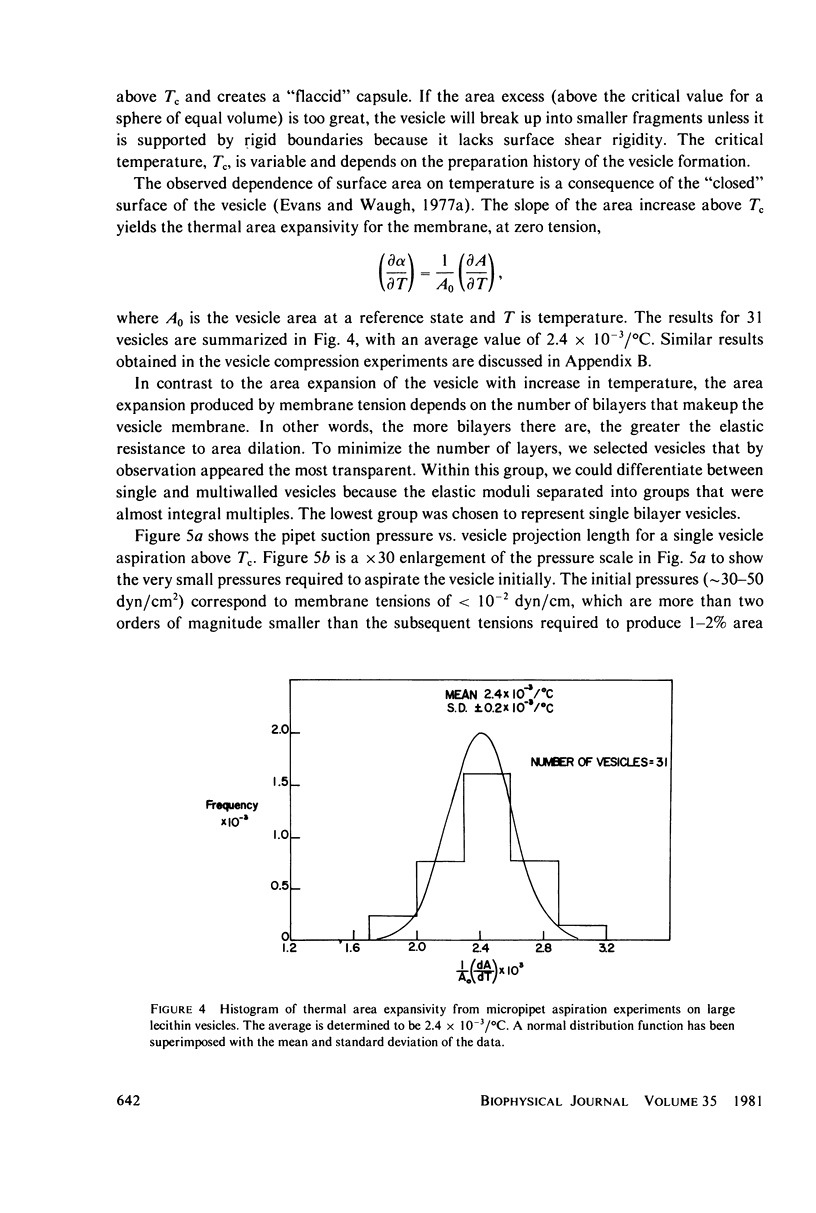
Abstract
Micromechanical experiments on large lecithin bilayer vesicles as a function of temperature have demonstrated an essential feature of bilayer vesicles as closed systems: the bilayer can exist in a tension-free state (within the limits of experimental resolution, i.e., less than 10(-2) dyn/cm). Furthermore, because of the fixed internal volume, there is a critical temperature at which the vesicle becomes a tension-free sphere. Below this temperature, thermoelastic tension builds up in the membrane and the vesicle's internal pressure increases while the surface area remains constant. Above this temperature, the vesicle's surface area increases while the tension and internal pressure are negligible. Without mechanical support, the vesicles fragment into small vesicles because they have insufficient surface rigidity. In the upper temperature range we have measured the increase of surface area with temperature. These data established the thermal area expansivity to be 2.4 X 10(-3)/degrees C. At constant temperature, we used either pipet aspiration with suction pressures up to 10(4) dyn/cm2 or compression against a flat surface with forces up to 10(-2) dyn to produce area dilation of the vesicle surface on the order of 1%. The rate of increase of membrane tension with area dilation was calculated, which established the elastic area compressibility modulus to be 140 dyn/cm. The tension limit that produced lysis was observed to be 3-4 dyn/cm (equivalent to 2-3% area increase). The product of the elastic area compressibility modulus, the thermal area expansivity, and the temperature gives the reversible heat of expansion at constant temperature for the bilayer. This value is 100 ergs/cm2 at 25 degrees C, or approximately 5 kcal/mol of lecithin. Similarly, the product of the thermal area expansivity multiplied by the area compressibility modulus determines the rate of increase of thermoelastic tension with decrease in temperature when the area is held constant, i.e., -0.34 dyn/cm/degrees C.
Full text
PDF


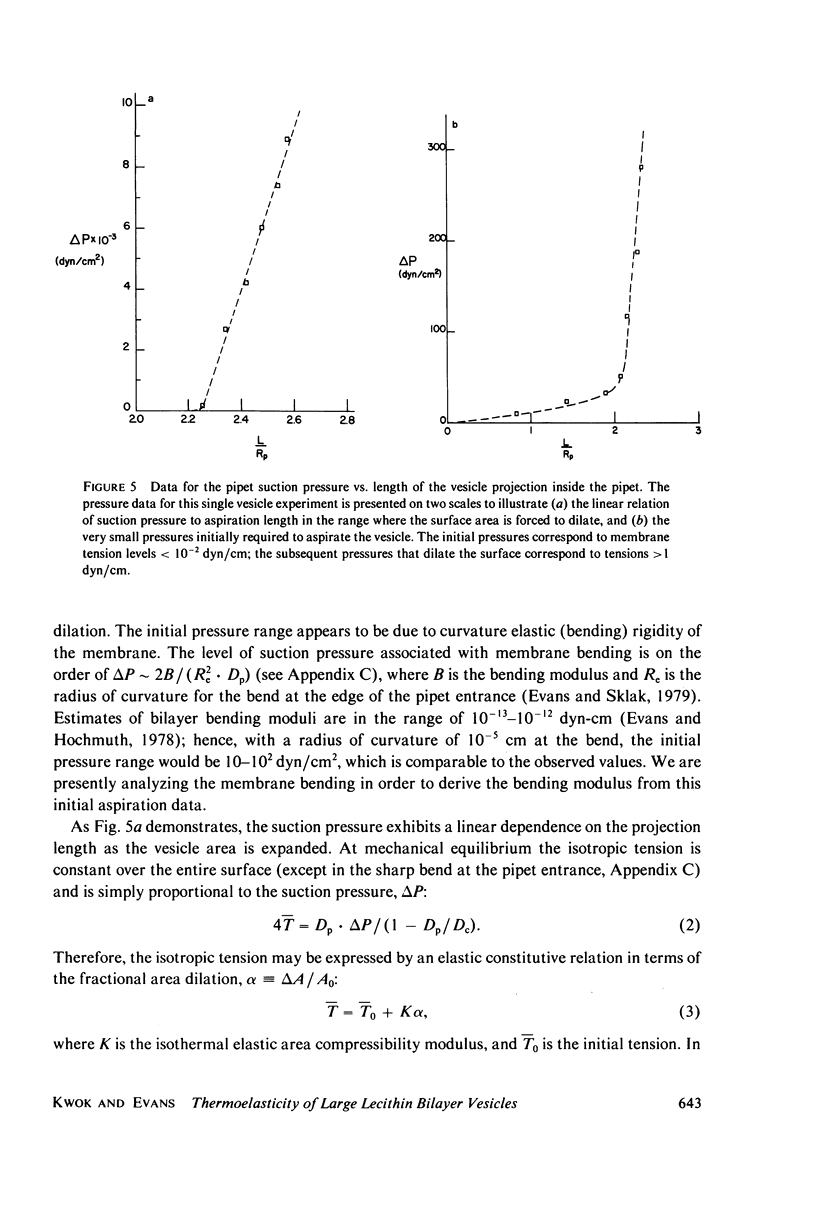
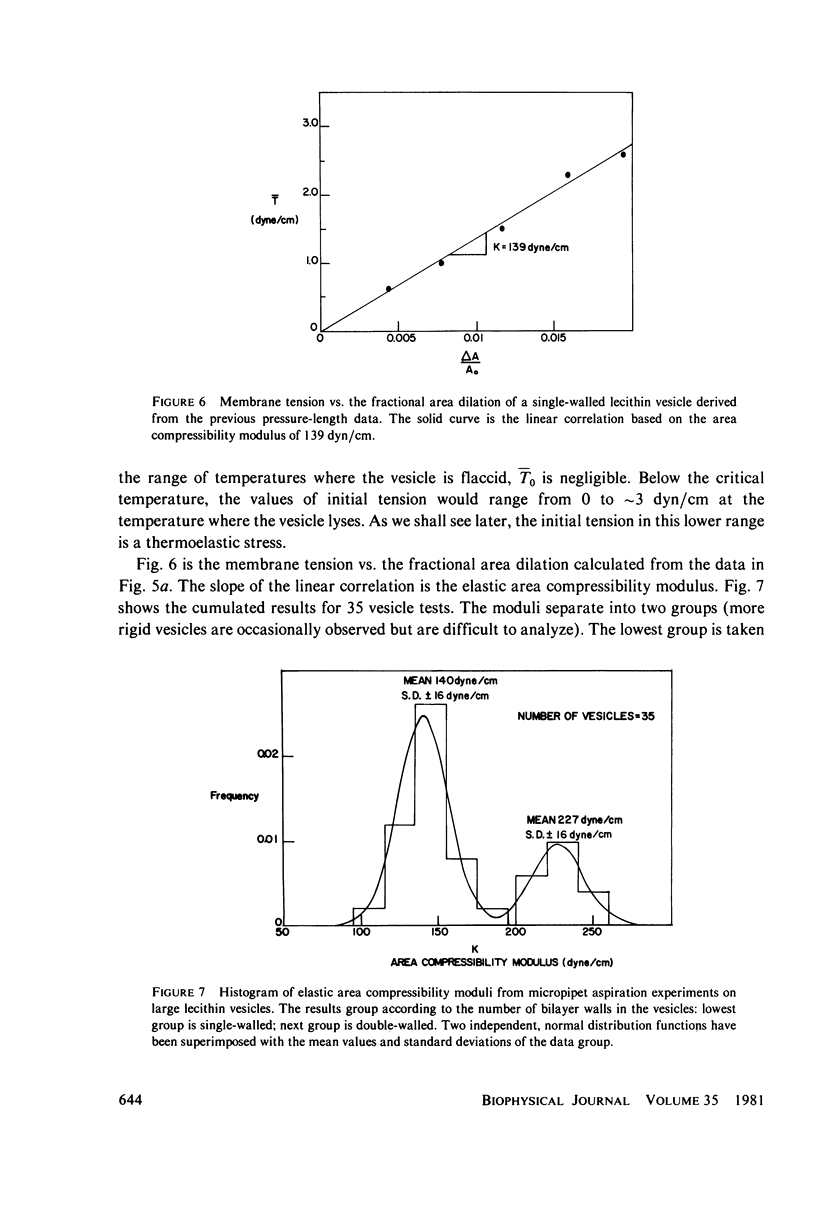
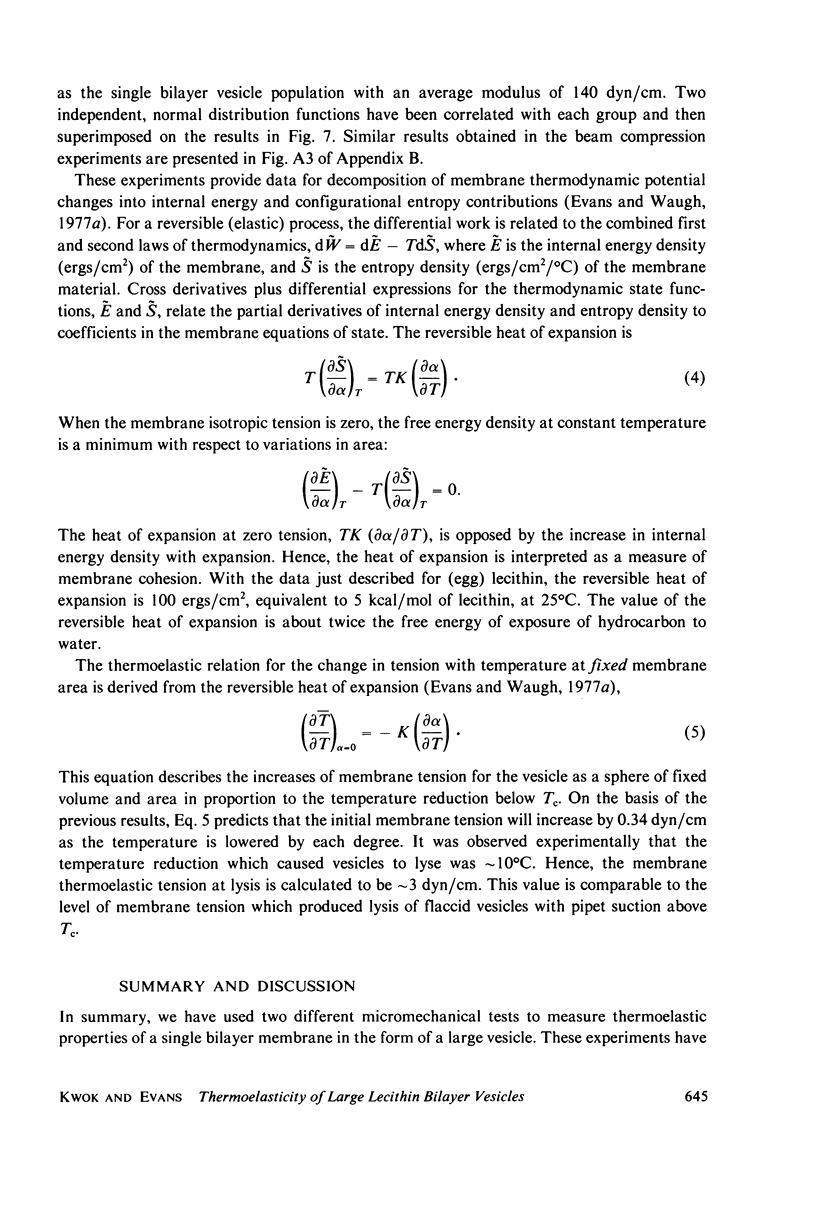



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez O., Latorre R. Voltage-dependent capacitance in lipid bilayers made from monolayers. Biophys J. 1978 Jan;21(1):1–17. doi: 10.1016/S0006-3495(78)85505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley J. M. Electrical breakdown of bimolecular lipid membranes as an electromechanical instability. Biophys J. 1973 Jul;13(7):711–724. doi: 10.1016/S0006-3495(73)86017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Kwok R., McCown T. Calibration of beam deflection produced by cellular forces in the 10(-9)--10(-6) gram range. Cell Biophys. 1980 Jun;2(2):99–112. doi: 10.1007/BF02795837. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Waugh R., Melnik L. Elastic area compressibility modulus of red cell membrane. Biophys J. 1976 Jun;16(6):585–595. doi: 10.1016/S0006-3495(76)85713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Waugh R. Osmotic correction to elastic area compressibility measurements on red cell membrane. Biophys J. 1977 Dec;20(3):307–313. doi: 10.1016/S0006-3495(77)85551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P. Measurement and modification of forces between lecithin bilayers. Biophys J. 1977 May;18(2):209–230. doi: 10.1016/S0006-3495(77)85608-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letter: Lenses and the compression of black lipid membranes by an electric field. Biophys J. 1975 Jan;15(1):77–81. doi: 10.1016/S0006-3495(75)85793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N. I., Kay R. L. Redetermination of the pressure dependence of the lipid bilayer phase transition. Biochemistry. 1977 Jul 26;16(15):3484–3486. doi: 10.1021/bi00634a030. [DOI] [PubMed] [Google Scholar]
- Macdonald A. G. A dilatometric investigation of the effects of general anaesthetics, alcohols and hydrostatic pressure on the phase transition in smectic mesophases of dipalmitoyl phosphatidylcholine. Biochim Biophys Acta. 1978 Feb 2;507(1):26–37. doi: 10.1016/0005-2736(78)90371-1. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Wilkinson D. A. Lecithin bilayers. Density measurement and molecular interactions. Biophys J. 1978 Aug;23(2):159–175. doi: 10.1016/S0006-3495(78)85441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. P., Dowben R. M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969 Feb;73(1):49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- Tien H. T. Black lipid membranes in aqueous media: interfacial free energy measurements and effect of surfactants on film formation and stability. J Phys Chem. 1967 Oct;71(11):3395–3401. doi: 10.1021/j100870a006. [DOI] [PubMed] [Google Scholar]
- Waugh R., Evans E. A. Thermoelasticity of red blood cell membrane. Biophys J. 1979 Apr;26(1):115–131. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H., Thompson T. E. Capacitance, area, and thickness variations in thin lipid films. Biochim Biophys Acta. 1973 Sep 27;323(1):7–22. doi: 10.1016/0005-2736(73)90428-8. [DOI] [PubMed] [Google Scholar]