Abstract
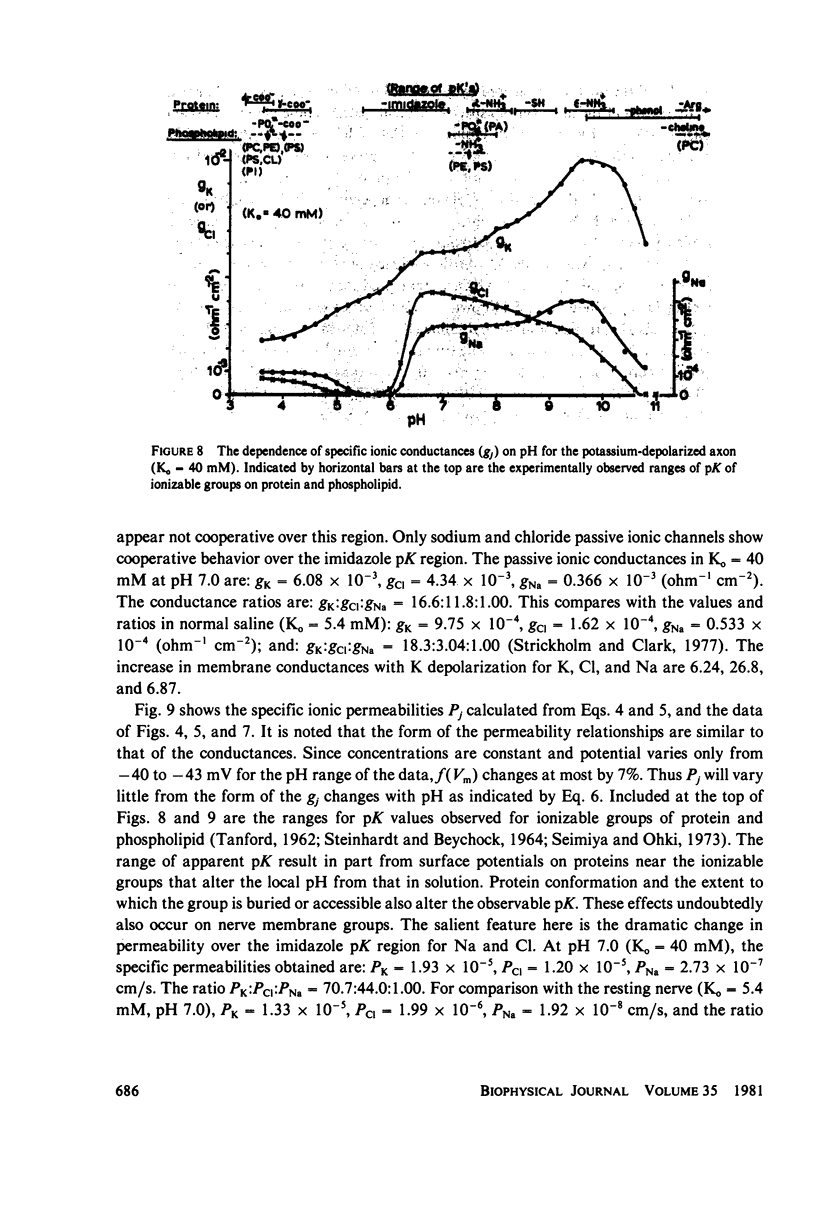
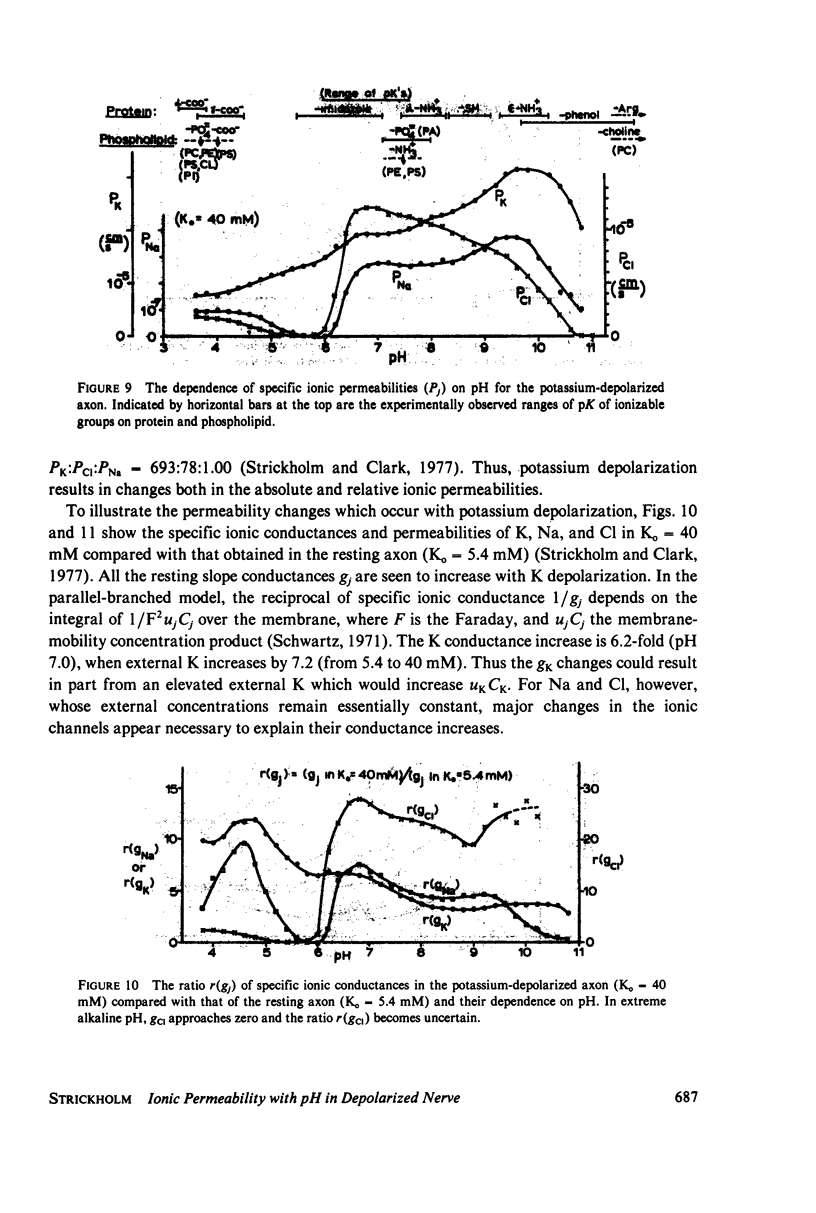
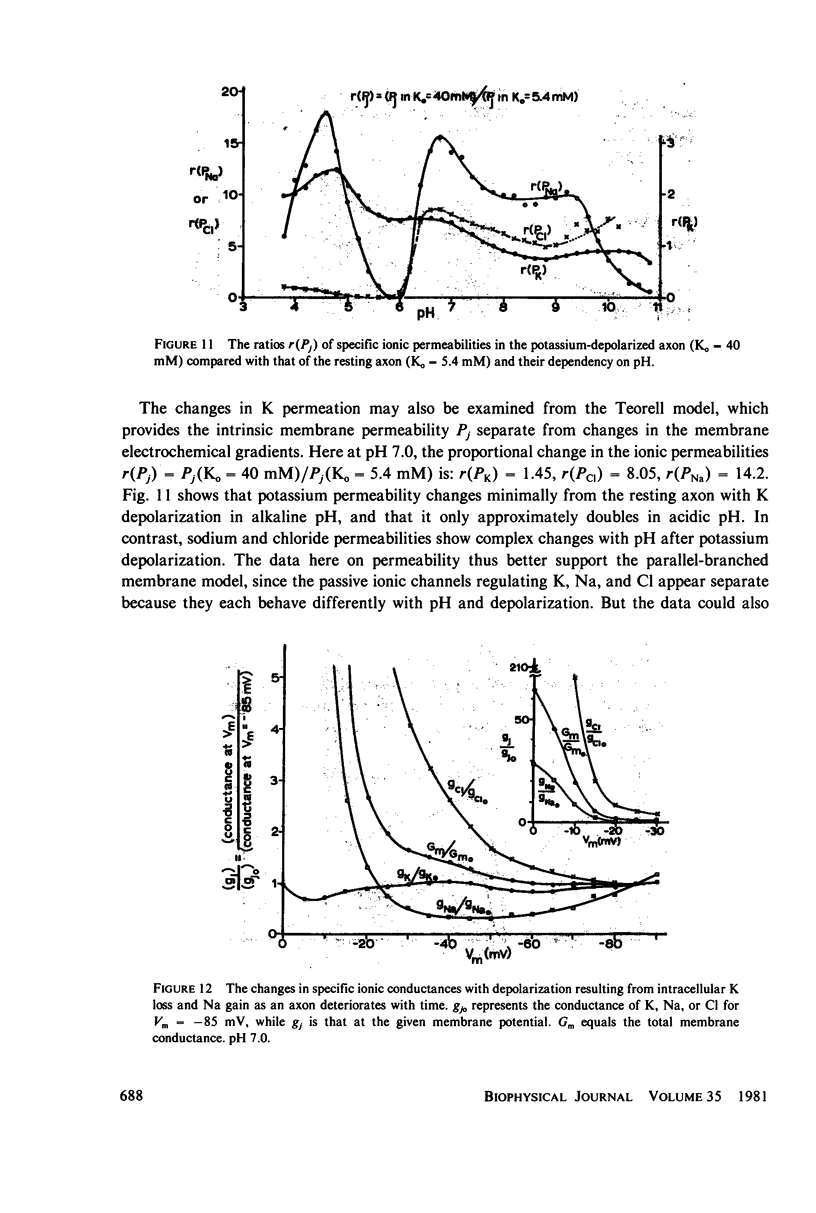
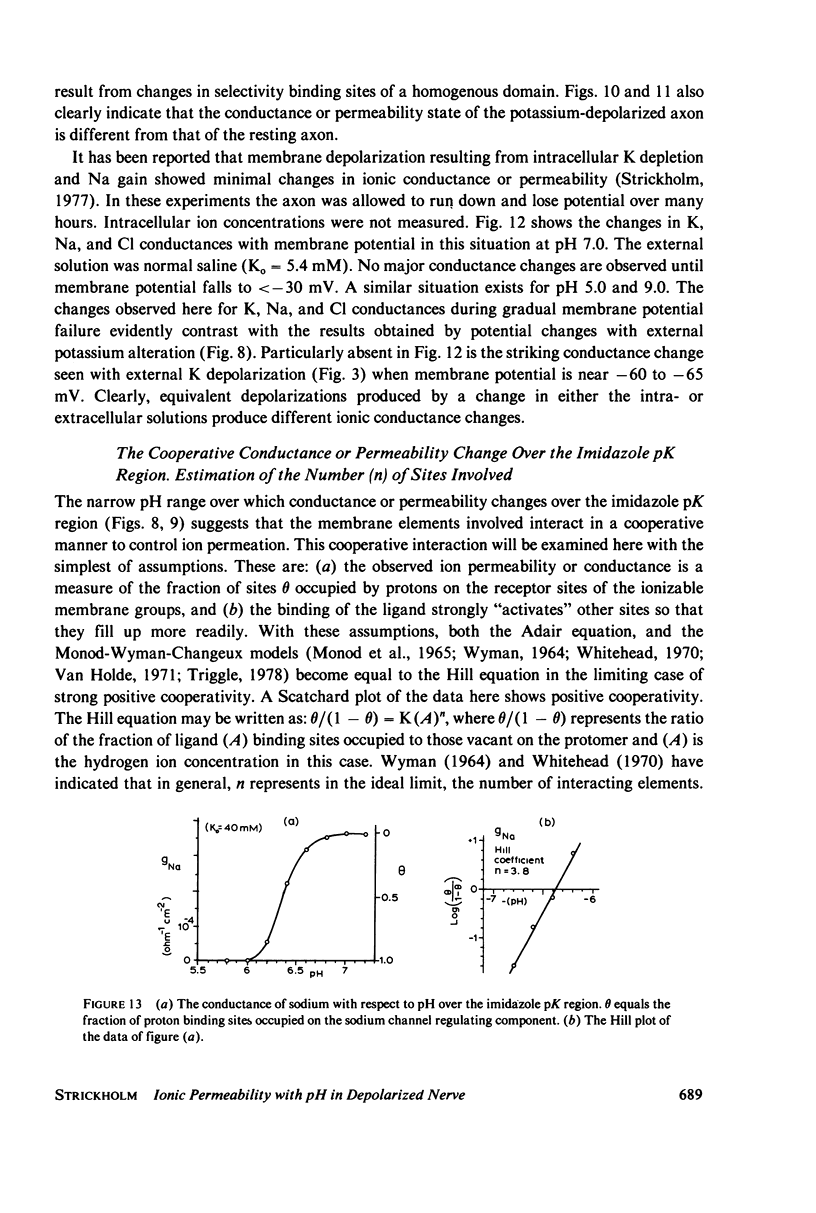
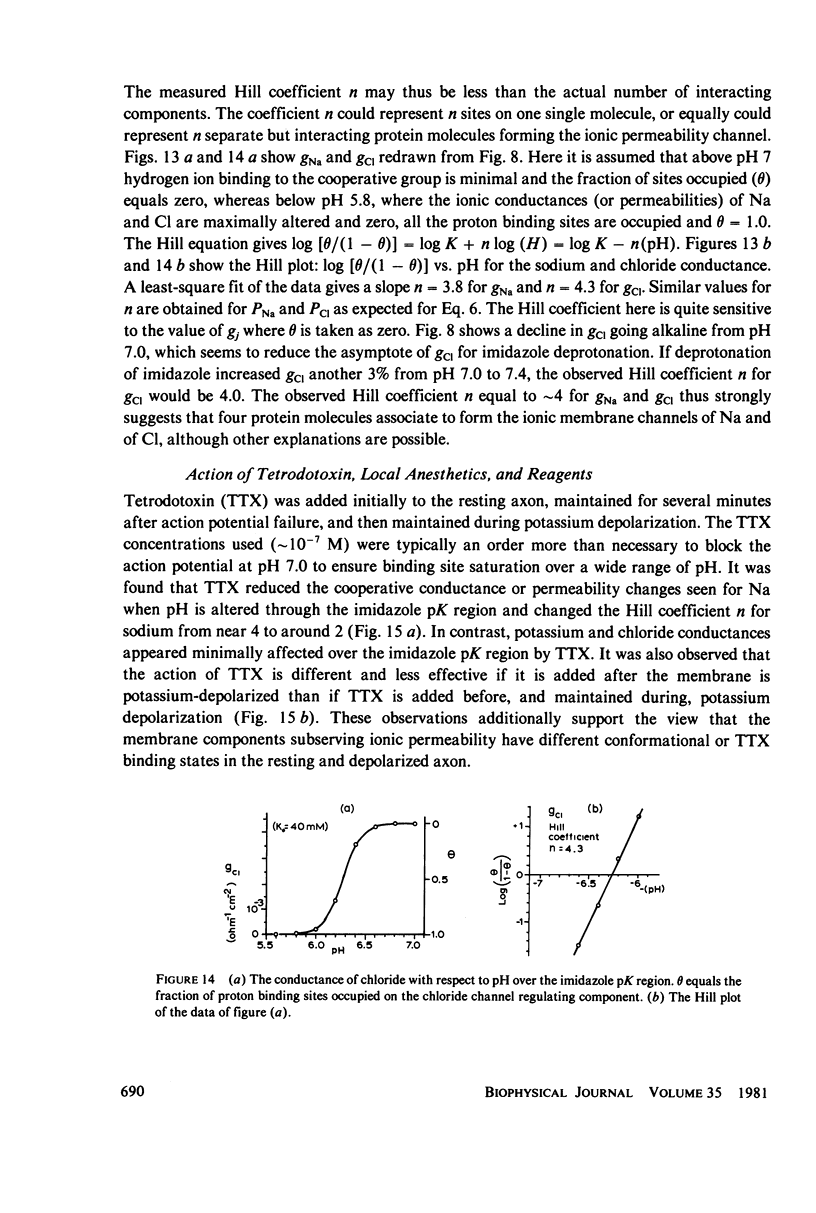
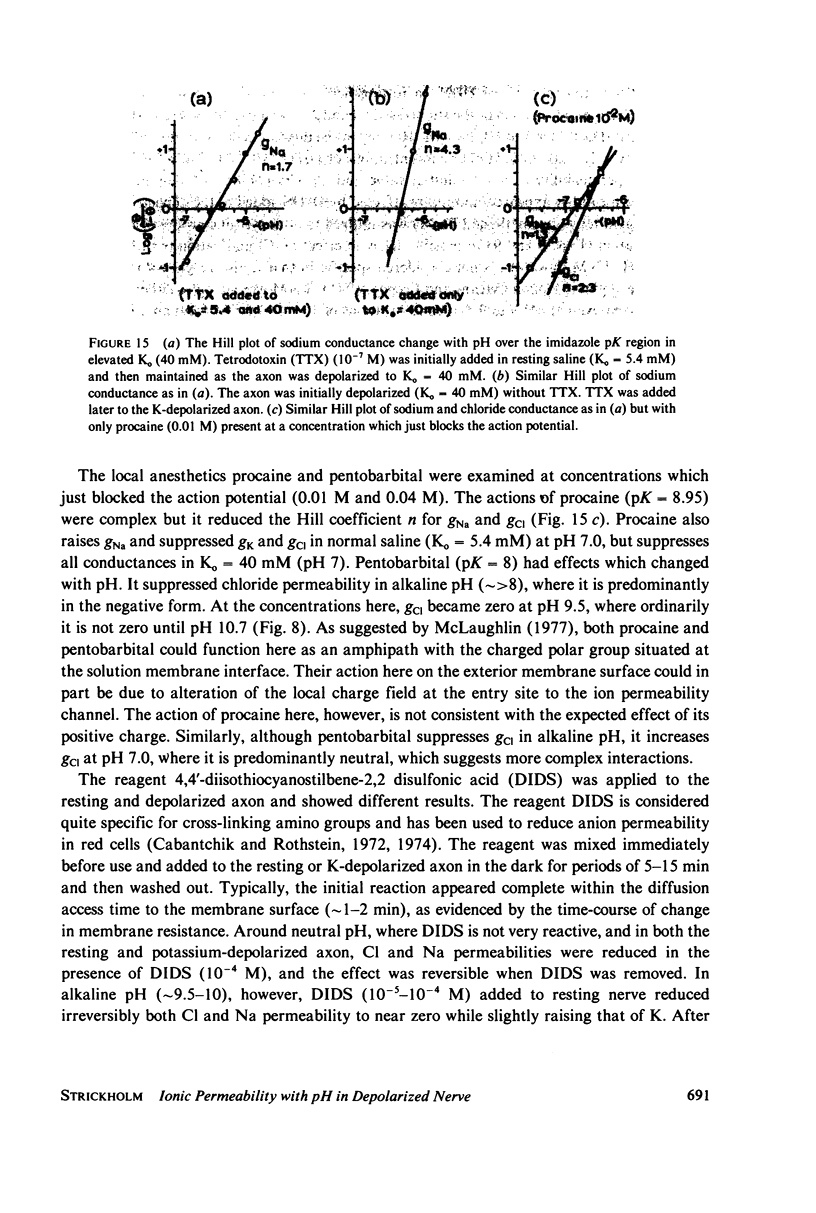
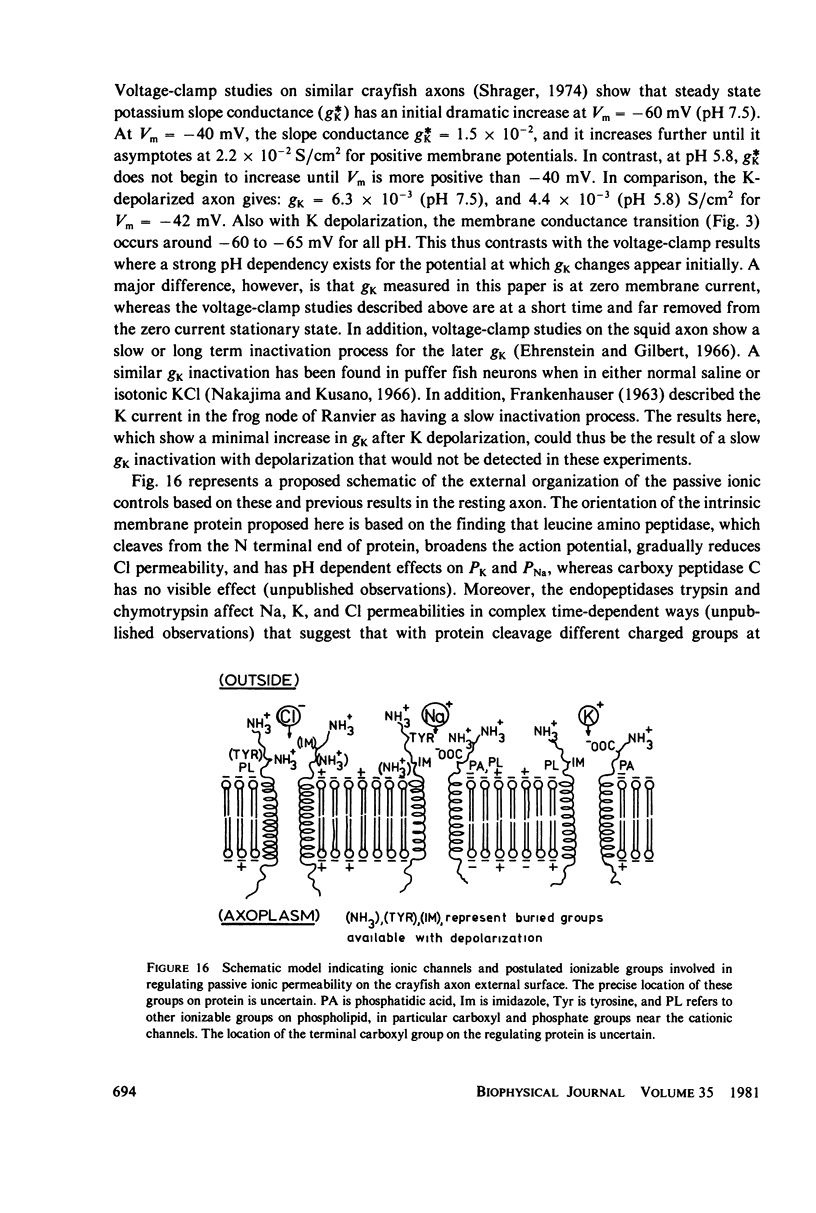
The passive ionic membrane conductances (gj) and permeabilities (Pj) of K, Na, and Cl of crayfish (Procambarus clarkii) medial giant axons were determined in the potassium-depolarized axon and compared with that of the resting axon. Passive ionic conductances and permeabilities were found to be potassium dependent with a major conductance transition occurring around an external K concentration of 12-15 mM (Vm = -60 to -65 mV). The results showed that K, Na, and Cl conductances increased by 6.2, 6.9, and 27-fold, respectively, when external K was elevated from 5.4 to 40 mM. Permeability measurements indicated that K changed minimally with K depolarization while Na and Cl underwent an order increase in permeability. In the resting axon (K0 = 5.4 mM, pH = 7.0) PK = 1.33 X 10(-5), PCl = 1.99 X 10(-6), PNa = 1.92 X 10(-8) while in elevated potassium (K0 = 40 mM, pH 7.0), PK = 1.9 X 10(-5), PCl = 1.2 X 10(-5), and PNa = 2.7 X 10(-7) cm/s. When membrane potential is reduced to 40 mV by changes in internal ions, the conductance changes are initially small. This suggests that resting channel conductances depend also on ion environments seen by each membrane surface in addition to membrane potential. In elevated potassium, K, Na, and Cl conductances and permeabilities were measured from pH 3.8 to 11 in 0.2 pH increments. Here a cooperative transition in membrane conductance or permeability occurs when pH is altered through the imidazole pK (approximately pH 6.3) region. This cooperative conductance transition involves changes in Na and Cl but not K permeabilities. A Hill coefficient n of near 4 was found for the cooperative conductance transition of both the Na and Cl ionic channel which could be interpreted as resulting from 4 protein molecules forming each of the Na and Cl ionic channels. Tetrodotoxin reduces the Hill coefficient n to near 2 for the Na channel but does not affect the Cl channel. In the resting or depolarized axon, crosslinking membrane amino groups with DIDS reduces Cl and Na permeability. Following potassium depolarization, buried amino groups appear to be uncovered. The data here suggest that potassium depolarization produces a membrane conformation change in these ionic permeability regulatory components. A model is proposed where membrane protein, which forms the membrane ionic channels, is oriented with an accessible amino terminal group on the axon exterior. In this model the ionizable groups on protein and phospholipid have varied associations with the different ionic channel access sites for K, Na, and Cl, and these groups exert considerable control over ion permeation through their surface potentials.
Full text
PDF
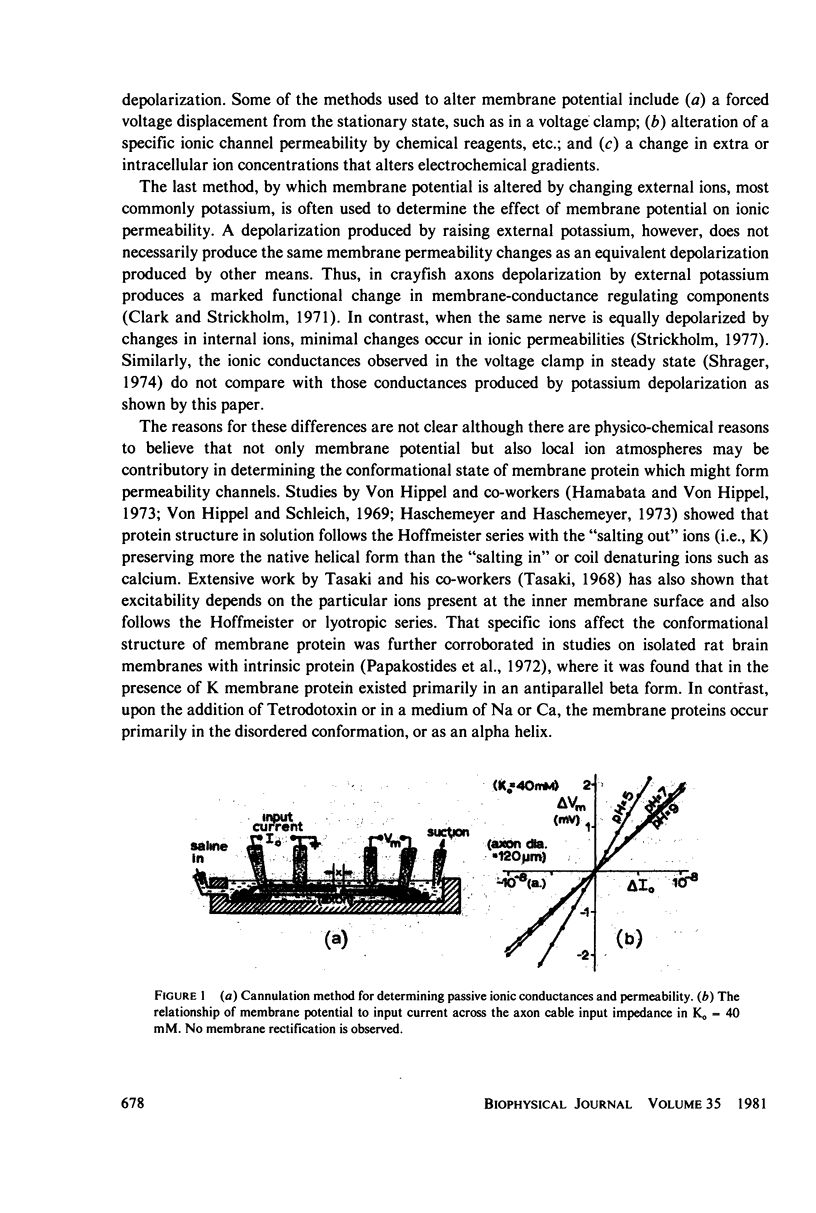


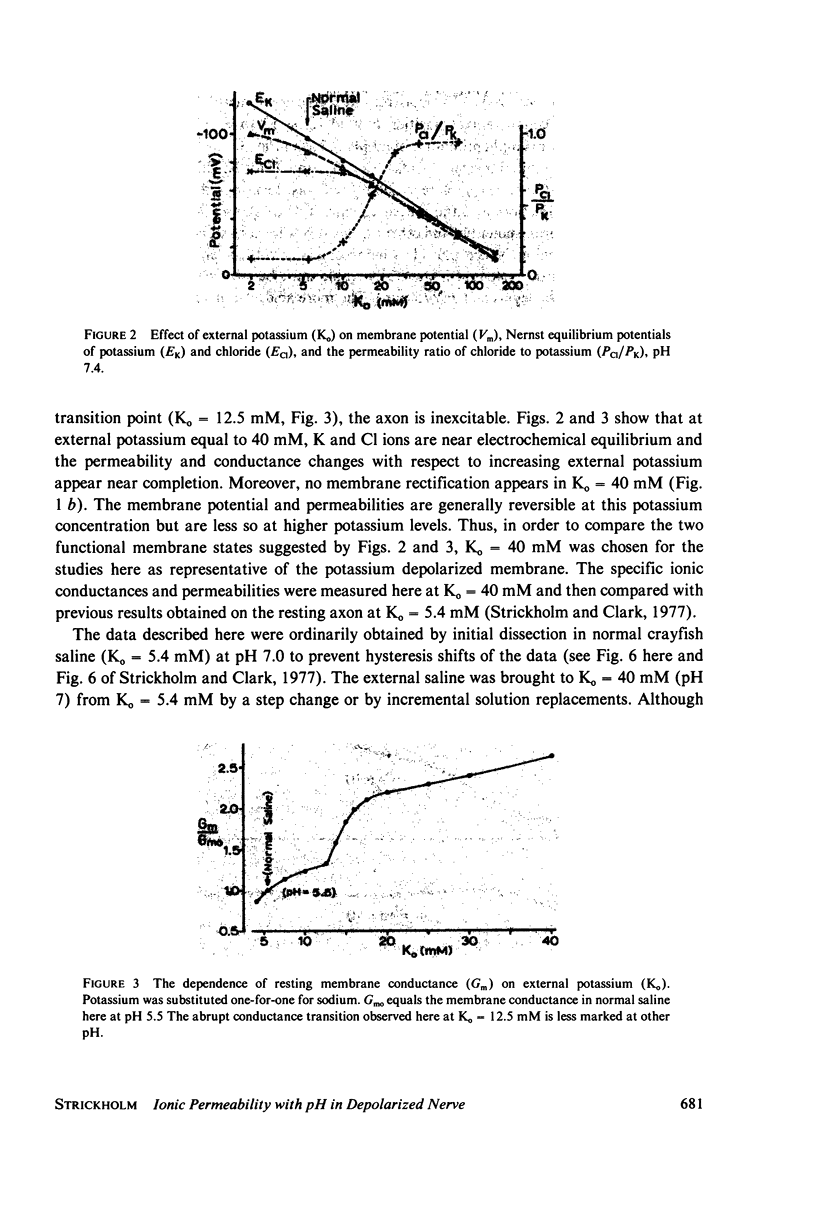
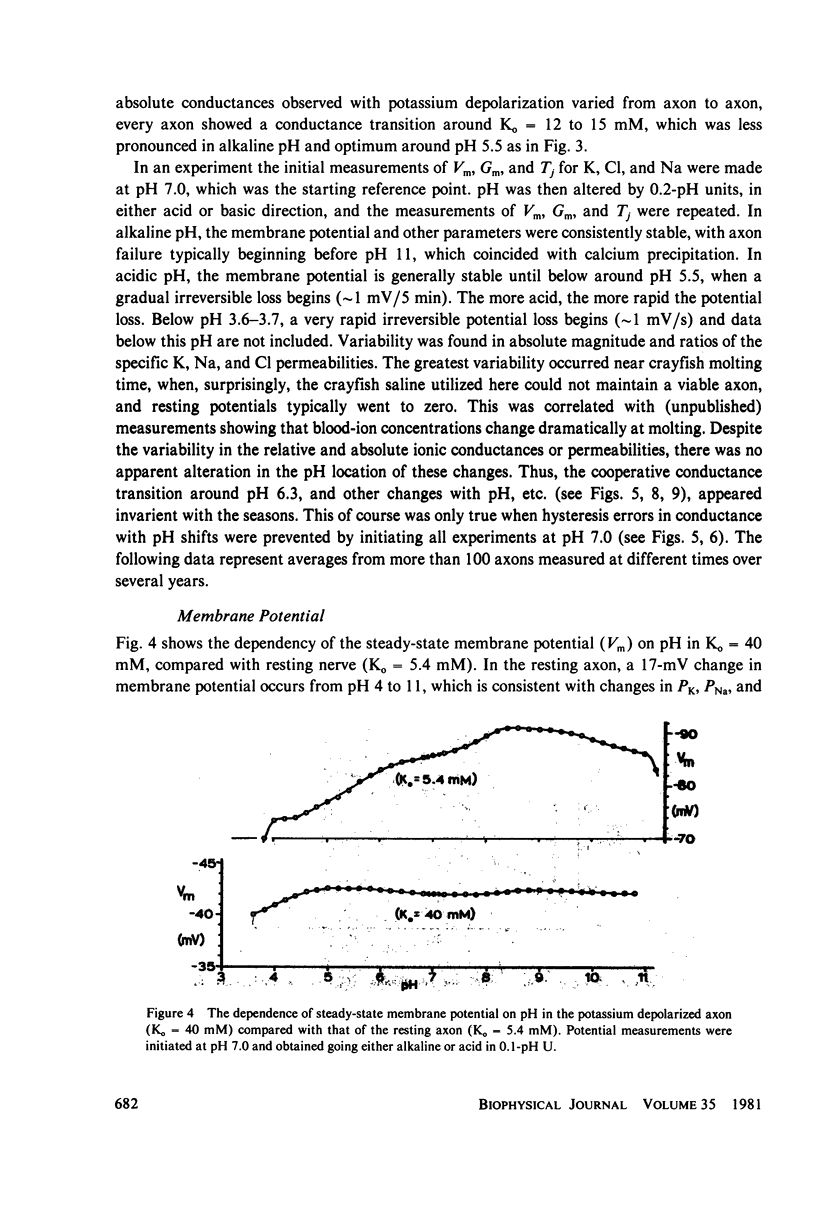

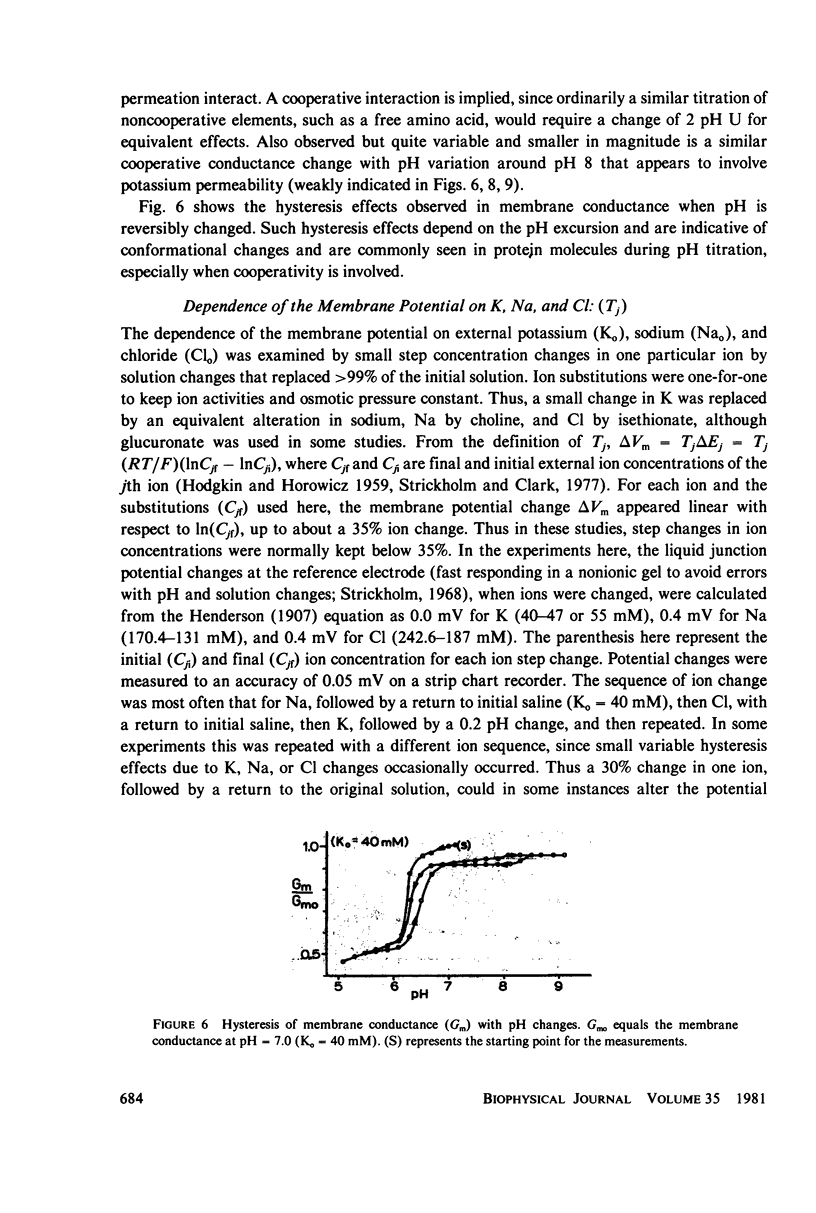






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Jr, Getting P. A., Thompson S. H. Mechanism of frequency-dependent broadening of molluscan neurone soma spikes. J Physiol. 1979 Jun;291:531–544. doi: 10.1113/jphysiol.1979.sp012829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. II. Effects of proteolytic enzymes on disulfonic stilbene sites of surface proteins. J Membr Biol. 1974;15(3):227–248. doi: 10.1007/BF01870089. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. Calcium-dependent depression of a late outward current in snail neurons. Science. 1977 Jul 29;197(4302):472–475. doi: 10.1126/science.17921. [DOI] [PubMed] [Google Scholar]
- Ehrenstein G., Gilbert D. L. Slow changes of potassium permeability in the squid giant axon. Biophys J. 1966 Sep;6(5):553–566. doi: 10.1016/S0006-3495(66)86677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyring H., Woodbury J. W., D'Arrigo J. S. A molecular mechanism of general anesthesia. Anesthesiology. 1973 May;38(5):415–424. doi: 10.1097/00000542-197305000-00001. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Mauro A. Equivalent Circuits as Related to Ionic Systems. Biophys J. 1963 May;3(3):215–237. doi: 10.1016/s0006-3495(63)86817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamabata A., Von Hippel P. H. Model studies on the effects of neutral salts on the conformational stability of biological macromolecules. II. Effects of vicinal hydrophobic groups on the specificity of binding of ions to amide groups. Biochemistry. 1973 Mar 27;12(7):1264–1271. doi: 10.1021/bi00731a004. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hladky S. B. Ion transport across thin lipid membranes: a critical discussion of mechanisms in selected systems. Q Rev Biophys. 1972 May;5(2):187–282. doi: 10.1017/s0033583500000883. [DOI] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Marquis J. K., Mautner H. G. The effect of electrical stimulation on the action of sulfhydryl reagents in the giant axon of squid: suggested mechanisms for the role of thiol and disulfide groups in electrically-induced conformational changes. J Membr Biol. 1974;15(3):249–260. doi: 10.1007/BF01870090. [DOI] [PubMed] [Google Scholar]
- Mozhaeva G. N., Naumov A. P. Vliianie poverkhnostnogo zariada na statsionarnuiu kalievuiu provodimost' membrany perekhvata Ranv'e. I. Izmenenie pH vneshnego rastvora. Biofizika. 1972 May-Jun;17(3):412–420. [PubMed] [Google Scholar]
- Papakostidis G., Zundel G., Mehl E. Na + -K + -dependent conformation change of proteins of excitable membranes. Biochim Biophys Acta. 1972 Nov 2;288(2):277–281. doi: 10.1016/0005-2736(72)90248-9. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Seimiya T., Ohki S. Ionic structure of phospholipid membranes, and binding of calcium ions. Biochim Biophys Acta. 1973 Mar 29;298(3):546–561. doi: 10.1016/0005-2736(73)90073-4. [DOI] [PubMed] [Google Scholar]
- Shrager P. Ionic conductance changes in voltage clamped crayfish axons at low pH. J Gen Physiol. 1974 Dec;64(6):666–690. doi: 10.1085/jgp.64.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickholm A., Clark H. R. Ionic permeability of K, Na, and Cl in crayfish nerve. Regulation by membrane fixed charges and pH. Biophys J. 1977 Jul;19(1):29–48. doi: 10.1016/S0006-3495(77)85560-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickholm A. Reduction of response time for potential in salt bridge reference electrodes for electrophysiology. Nature. 1968 Jan 6;217(5123):80–81. doi: 10.1038/217080a0. [DOI] [PubMed] [Google Scholar]
- Strickholm A., Wallin B. G. Relative ion permeabilities in the crayfish giant axon determined from rapid external ion changes. J Gen Physiol. 1967 Aug;50(7):1929–1953. doi: 10.1085/jgp.50.7.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W. Ionic channels and gating currents in excitable membranes. Annu Rev Biophys Bioeng. 1977;6:7–31. doi: 10.1146/annurev.bb.06.060177.000255. [DOI] [PubMed] [Google Scholar]
- WYMAN J., Jr LINKED FUNCTIONS AND RECIPROCAL EFFECTS IN HEMOGLOBIN: A SECOND LOOK. Adv Protein Chem. 1964;19:223–286. doi: 10.1016/s0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- Wallin B. G. The relation between external potassium concentration, membrane potential and internal ion concentrations in crayfish axons. Acta Physiol Scand. 1967 Jul-Aug;70(3):431–448. doi: 10.1111/j.1748-1716.1967.tb03641.x. [DOI] [PubMed] [Google Scholar]
- Whitehead E. The regulation of enzyme activity and allosteric transition. Prog Biophys Mol Biol. 1970;21:321–397. doi: 10.1016/0079-6107(70)90028-3. [DOI] [PubMed] [Google Scholar]


