Abstract
The objective of the present study was to investigate the potential bactericidal activity of amoxicillin-clavulanate against β-lactamase-producing Escherichia coli strains and to elucidate the extent to which enzyme production affects the activity. Six adult Yucatan miniature pigs received a single intravenous dose of 1.1 g of amoxicillin-clavulanate as an intravenous infusion over 30 min. The pharmacokinetic parameters were determined for the serum samples and compared to the published data for humans (2.2-g intravenous dose). The parameters were comparable for the two species, and therefore, the miniature pig constitutes a good model for pharmacodynamic study of amoxicillin-clavulanate. Therefore, the model was used in an ex vivo pharmacodynamic study of amoxicillin-clavulanate against four strains of Escherichia coli producing β-lactamases at different levels. The E. coli strains were cultured with serial dilutions (1:2 to 1:256) of the serum samples from the pharmacokinetic study, and the number of surviving bacteria was determined after 1, 3, and 6 h of exposure. Amoxicillin-clavulanate at concentrations less than the MIC and the minimal bactericidal concentration had marked bactericidal potency against the strain that produced low levels of penicillinase. For high-level or intermediate-level β-lactamase-producing strains, the existence of a clavulanate concentration threshold of 1.5 to 2 μg/ml, below which there was no bactericidal activity, was demonstrated. The index of surviving bacteria showed the existence of mixed concentration- and time-dependent actions of amoxicillin (in the presence of clavulanate) which varied as a function of the magnitude of β-lactamase production by the test strains. This study shows the effectiveness of amoxicillin-clavulanate against low- and intermediate-level penicillinase-producing strains of E. coli. These findings are to be confirmed in a miniature pig experimental infection model.
Amoxicillin-clavulanate is a combination of a β-lactam with a β-lactamase inhibitor which restores the potency of amoxicillin against strains producing β-lactamases. The potential for the clinical use of such combinations depends in part on the concentrations achieved in serum and tissue after the administration of the usual dosages compared to the MIC for the infecting organism. In particular, it is necessary to obtain concentrations sufficiently inhibitory for the β-lactamase in vivo to inactivate the enzymes produced by the bacteria at the infection site.
The sensitivity of Escherichia coli to the combination of amoxicillin plus clavulanate depends on the strain's level of β-lactamase production. The number of resistant or intermediate-resistant strains is increasing (1); and in E. coli overproduction of chromosomal β-lactamases of class C (cephalosporinase) or plasmid enzymes, e.g., TEM-1, TEM-2, and OXA (1, 3, 23), is the most common mode of β-lactam resistance.
The aim of the present study was to evaluate the bactericidal potency of amoxicillin-clavulanate in an ex vivo bactericidal model using the Yucatan miniature pig.
The miniature pig is physiologically similar to humans (17) and shows similarities to humans in terms of the pharmacokinetics of several β-lactams (cefepime, cefpirome, and meropenem) and other xenobiotics (6). The animal is docile and, because it is small, easy to handle (8). In addition, it is feasible to take repeated samples of sufficient volume to enable pharmacokinetic studies.
Initially, we studied the pharmacokinetics of amoxicillin-clavulanate administered intravenously (i.v.) at 1.1 g to miniature pigs as a 30-min infusion in order to compare the results with those obtained for humans following administration of 2.2 g i.v. Subsequently, using that model, we conducted an ex vivo pharmacodynamic study of amoxicillin-clavulanate using four strains of E. coli producing β-lactamases at different levels.
MATERIALS AND METHODS
Miniature pigs.
Adult Yucatan miniature pigs were supplied by the Charles River Company (St-Aubin-les-Elbeuf, France). Six miniature pigs (females) were used in the pharmacokinetic and pharmacodynamic studies. The body weights ranged from 16 to 25 kg (mean, 22.2 ± 3.4 kg). Each animal was placed in an individual pen in the same room. The diet consisted of a special miniature pig diet (UAR, Epinay-sur-Orge, France) that does not contain antibiotics or any other drug. The handling required for the kinetic studies was conducted with conscious animals constrained in a special hammock (Panepinto model; Charles River) (18).
The experimental procedures were in accordance with the recommendations of the French Ministry of Forest and Agriculture for the use of laboratory animals. Permission (A67482-11) to conduct experiments with the animals was given by the French Ministry of Forest and Agriculture.
Catheterization.
Fasting animals were premedicated by intramuscular administration of 2.5 mg of droperidol (2.5 mg/ml; Droleptan; Janssen-Cilag) and then received general anesthesia by halothane inhalation and propofol (4 mg/kg of body weight) by i.v. administration in order to catheterize the external jugular. The catheter was lodged in a tunnel and exited in a dorsal medial sagittal position, as described previously (2, 9, 20).
Antibiotics.
The human formulation of amoxicillin-clavulanate for i.v. injection was supplied by SmithKline Beecham (Heppignies, Belgium), as were amoxicillin and clavulanate titrated powder. Titrated piperacillin powder was supplied by Sanofi Winthrop (Gentilly, France).
Antibiotic assay.
Serum amoxicillin and clavulanate concentrations were determined by high-performance liquid chromatography by a previously reported protocol (12, 13, 15). Briefly, both drugs were analyzed on a reversed-phase analytical column (System Gold high-performance liquid chromatograph; Beckman-Coulter, Fullerton, Calif.). The samples were run in a high-speed Ultrasphere C18, 3-μm spherical 80-Å-pore-size column (75 by 4.6 mm; Beckman-Coulter). The mobile phase for clavulanic acid consisted of 8% acetonitrile in 3 mM tetraethylammonium bromide, 2 mM octanesulfonic acid, and 25 mM phosphate buffer (pH 6.5). The final pH was adjusted to 2 with orthophosphoric acid. The flow rate was set at 1 ml/min, and detection occurred at 315 nm. Fifty microliters of the extracted sample (15), derivatized with 10 μl of imidazole, was injected into the chromatograph. The intraday coefficients of variation were as follows: for a concentration of 0.01 μg/ml, 1.3% (n = 10); for a concentration of 0.10 μg/ml, 2.3% (n = 10); and for a concentration of 1.00 μg/ml, 1.3% (n = 10). The interday coefficients of variation were 3.9, 3.4, and 3.3% for the three concentrations, respectively. For amoxicillin, the mobile phase consisted of 13% acetonitrile in 5 mM tetrabutylammonium bromide and 15 mM ammonium acetate. The pH was adjusted to 7.5 with natrium hydroxide. The flow rate was set at 1 ml/min, and detection occurred at 227 nm. Fifty microliters of the sample, which was extracted from serum (15), was directly injected into the chromatograph. The intraday coefficients of variation were as follows: for a concentration of 0.5 μg/ml, 4.0% (n = 9); for a concentration of 10.0 μg/ml, 1.8% (n = 9); and for a concentration of 100.0 μg/ml, 3.0% (n = 9). The interday coefficients of variation were 5.9, 2.6, and 3.4% for the three concentrations, respectively. The limit of detection for amoxicillin was 0.1 μg/ml, and that for clavulanate was 0.01 μg/ml.
Pharmacokinetic study.
A single dose of amoxicillin-clavulanate was injected through a short catheter that had been inserted for that purpose into an auricular vein. Given the body weights of the miniature pigs (16 to 25 kg), the amoxicillin-clavulanate dose administered was 1.1 g (1 g of amoxicillin plus 0.1 g of clavulanate), equivalent to half the standard dosage used in human clinical practice (2.2 g administered i.v.). Amoxicillin-clavulanate was diluted in normal saline in a 50-ml syringe. Amoxicillin-clavulanate was administered as an i.v. infusion over 30 min.
Blood samples were obtained from a three-way connector extension attached to the connector of the jugular catheter, enabling the first fraction of blood collected to be reinjected (purge) and thus minimizing the volume changes. The specimens (about 10 ml each) were taken preinfusion (control) and then at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 5, 8, and 12 h postinfusion. All the specimens were immediately centrifuged at 2,000 × g at 4°C for 10 min. The serum was stored at −80°C until analysis.
Pharmacokinetic analyses.
The standard kinetic parameters were determined as described by Gibaldi and Perrier (11). The area under the serum concentration-time curve (AUC) was determined by the trapezoidal method (Sigma Plot; SPSS Science, Chicago, Ill.). Total clearance was calculated as the ratio of the i.v. dose injected to the AUC and was expressed relative to the individual body weight of each miniature pig (7). The elimination half-life (t1/2) was estimated by linear regression (Sigma Plot).
The results were expressed as means ± standard deviations, and the parameters were compared with those published for humans for a dosage of 2.2 g of amoxicillin-clavulanate administered i.v. (21).
Bacterial strains.
The β-lactamase-producing E. coli strains used in the study had recently been isolated from patients presenting with urinary tract infections. The strains were selected on the basis of their degrees of resistance to amoxicillin, piperacillin, and amoxicillin-clavulanate. On the basis of the MICs of those three antibiotics, the strains were rated as low-level penicillinase-producing (LLP), intermediate-level penicillinase-producing (ILP), or high-level penicillinase-producing (HLP) strains.
In vitro sensitivities of the bacterial strains by MIC and MBC determinations.
The MICs of piperacillin and amoxicillin were determined by the reference microdilution method (4) in broth medium with drug concentrations over a range from 0.125 to 256 μg/ml. For amoxicillin-clavulanate MIC determination, 2 μg of clavulanate per ml was added to serial amoxicillin dilutions. The bacterial inoculum, prepared from a 3-h culture in the exponential growth phase, was diluted to obtain 106 to 107 CFU/ml. Briefly, each antibiotic was added to 1.8 ml of the inoculum in 20-fold concentrations from 2.5 to 5,120 μg/ml for amoxicillin and 40 μg/ml for clavulanate. The MICs were the lowest concentrations at which no visible growth was observed after 18 h of incubation at 37°C. For the control, the bacterial inoculum was serially diluted to 1/104, and 100 μl was transferred to a Mueller-Hinton (MH) agar plate.
The minimum bactericidal concentration (MBC) was determined by plating 100 μl from each tube without visible growth. The MBC was defined as the lowest concentration that reduced the CFU of the inoculum by at least 99.99%, corresponding to 0.01% survivors after 24 h of exposure.
Pharmacodynamic analysis.
The serum samples from the miniature pigs taken for the pharmacokinetic analysis were pooled at each time point in equal volumes to obtain sufficient volumes to enable the study of all bacterial strains, which means that all the pig serum samples from 0.25 h were pooled, all serum samples from 0.5 h were pooled, and so on up to 12 h.
Serial dilutions of the serum samples from 1:2 to 1:256 were prepared in MH broth enriched with 5% bovine albumin (8). A suspension of each of the test strains containing 106 CFU/ml was added. The final volume was 1 ml. The bactericidal kinetic study was conducted at 37°C.
Bacterial counts were measured from serial dilutions of the serum samples at time zero and then after 1, 3, and 6 h of exposure by manually transferring a volume of 50 μl to MH agar. Reading of the bacterial counts was conducted after 24 h of incubation at 37°C. The limit of detection of the bacterial counts was 20 CFU/ml.
Determination of ISB.
For each dilution of serum obtained from the pharmacokinetic study at 0.25 to 12 h and tested with the various strains, the resulting bactericidal kinetic data enabled calculation of the index of surviving bacteria (ISB), as described by Garraffo et al. (10). For each dilution, the ISB (in percent) was equal to the ratio of the area under the bacterial killing curve (surviving bacteria) following treatment in comparison with the hypothetical area under the growth curve of the initial inoculum if no bactericidal activity occurred: (AUC for surviving bacteria/AUC for initial inoculum) × 100.
The AUC of the bactericidal kinetics used in the calculation was determined by the trapezoidal method (Sigma Plot). This calculation was performed at 6 h of incubation. If no bactericidal effect was obtained, the AUC for the surviving bacteria was then above the AUC for the initial inoculum, and the ISB was above 100% and was not represented (see Fig. 4). A time-kill curve only allows specification of the degree of the decrease in the inoculum at a time (either 1, 3, or 6 h) when the ISB, which is the ratio of the whole curve on the inoculum curve, explores the global bactericidal activity between 0 and 12 h and further allows the evolution of ISB as a function of the antibiotic concentrations. The value of the slope obtained by correlating the ISB and the in vivo concentration of amoxicillin indicates the concentration or time dependence of the bactericidal activity. The more the slope is negative, the faster is the rate of killing and the greater is the dependence of the bactericidal activity on the concentration.
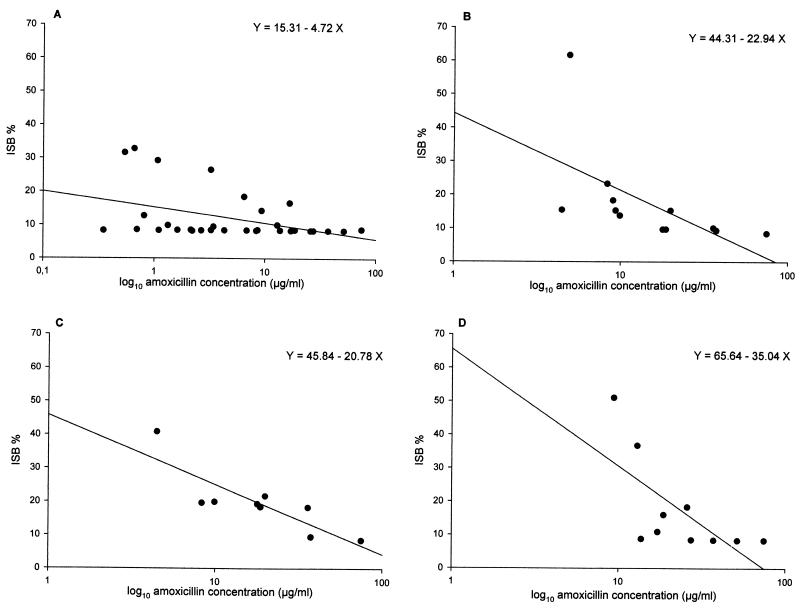
FIG. 4.
Relationship between ISB and amoxicillin concentrations for the E. coli strains tested after 6 h of contact. (A) LLP strain (amoxicillin-clavulanate MIC, 4 μg/ml); (B) ILP strain 8425 (amoxicillin-clavulanate MIC, 8 μg/ml); (C) ILP strain 425 (amoxicillin-clavulanate MIC, 16 μg/ml); (D) HLP strain (amoxicillin-clavulanate MIC, ≥256 μg/ml).
RESULTS
Pharmacokinetics.
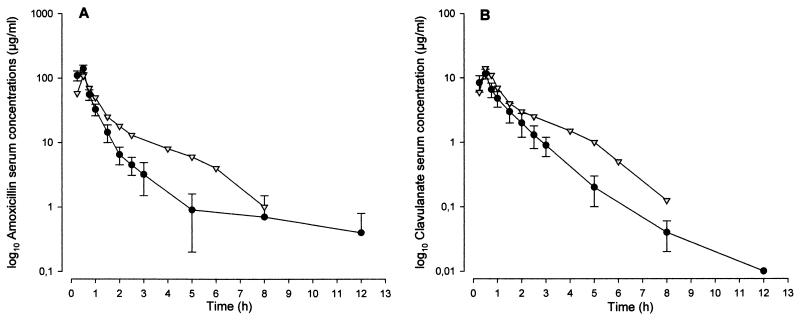
Figure 1 shows the mean ± standard deviation serum amoxicillin and clavulanate concentrations obtained as a function of time in the six miniature pigs following infusion of 1.1 g of amoxicillin-clavulanate over 30 min. The concentrations in human serum following infusion of 2.2 g over 30 min are shown for comparison.
FIG. 1.
Mean serum concentration-time curves for amoxicillin (A) and clavulanic acid (B) after a 30-min infusion of 1.1 g of amoxicillin-clavulanate i.v. to miniature pigs (•) and a 30-min infusion of 2.2 g of amoxicillin-clavulanate i.v. to humans (▿) (21).
The resulting pharmacokinetic parameters are shown in Table 1 and compared with those reported for humans in the study by Staniforth et al. (21). In the miniature pig, the mean ± standard deviation concentrations in serum obtained at the end of infusion were 139.7 ± 18.7 μg/ml for amoxicillin and 11.6 ± 2.0 μg/ml for clavulanate. The AUCs as a function of time were 108.3 ± 15.8 mg · h · liter−1 for amoxicillin and 13.2 ± 3.8 mg · h · liter−1 for clavulanate. The t1/2s, obtained from fitting of the elimination data, were 1.1 ± 0.5 h for amoxicillin (r2= 0.9261) and 0.9 ± 0.1 h for clavulanate (r2=0.9887).
TABLE 1.
Pharmacokinetics of amoxicillin and clavulanate after a 30-min infusion of amoxicillin-clavulanate at 1.1 g i.v.a to miniature pigs and comparison with the values for humansb
| Drug and subject | Dose (mg) | Cmax (μg/ml) | Tmax (h) | t1/2 (h) | AUCc (mg · h · liter−1) | Clearance (ml/min) |
|---|---|---|---|---|---|---|
| Amoxicillin | ||||||
| Miniature pigs | 1,000 | 139.7 ± 18.7 | 0.5 | 1.1 ± 0.5 | 108.3 ± 15.8 | 202 ± 35.2 |
| Humansd | 2,000 | 108.3 ± 20.6 | 0.5 | 1.0 | 125.0 | 240 |
| Clavulanate | ||||||
| Miniature pigs | 100 | 11.6 ± 2.0 | 0.5 | 0.9 ± 0.1 | 13.2 ± 3.8 | 178 ± 52.8 |
| Humansd | 200 | 13.8 ± 2.8 | 0.5 | 0.8 | 18.3 | 180 |
The dose corresponded to one of 2.2 g for humans.
Abbreviations: Cmax, maximum concentration in drug in serum; Tmax, time to maximum concentration of drug in serum.
From 0 to 8 h.
Data for humans are from Staniforth et al. (21).
Pharmacodynamics.
The MICs and MBCs for all E. coli strains tested are shown in Table 2.
TABLE 2.
Susceptibilities of the E. coli strains tested to amoxicillin-clavulanate, amoxicillin, and piperacillin, as determined by the microdilution methoda
| E. coli strain | MIC (μg/ml)
|
MBC (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| AMX | AMCb | PIP | AMX | AMCb | PIP | |
| LLP | >256 | 4 | 64 | >256 | 4 | 64 |
| ILP | ||||||
| 425 | >256 | 16 | >256 | >256 | 16 | >256 |
| 8425 | >256 | 8 | >256 | >256 | 16 | >256 |
| HLP | >256 | >256 | >256 | >256 | >256 | >256 |
Abbreviations: AMX, amoxicillin; AMC, amoxicillin-clavulante; PIP, piperacillin.
Tested with 2 μg of clavulanate per ml and expressed as the concentration of amoxicillin.
LLP strain.
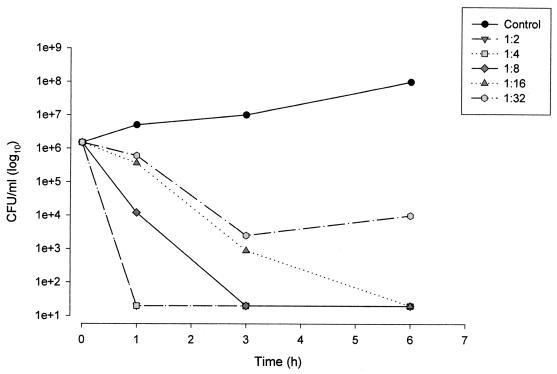
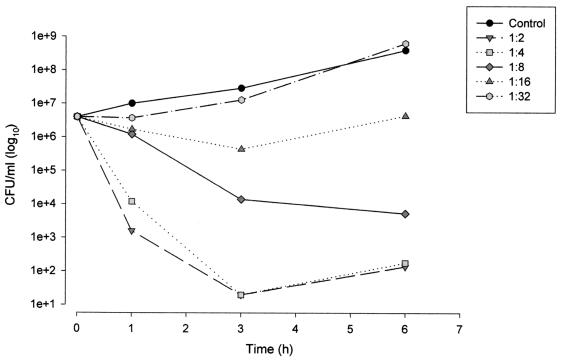
The ex vivo bactericidal kinetics for the various dilutions of serum sampled at the time of the peak concentration in serum (0.5 h) for the LLP strain are shown in Fig. 2. For the 1:2 and 1:4 dilutions, after 1 h of exposure, 5-log10 reductions in bacterial counts were observed. A bactericidal response was observed up to the 1:16 dilution (amoxicillin concentration, 8.7 μg/ml; clavulanate concentration, 0.7 μg/ml) following 6 h of exposure.
FIG. 2.
Kinetic killing curves for the LLP E. coli strain exposed to serial dilutions of serum (1:2 to 1:32) sampled 0.5 h after i.v. infusion of 1.1 g of amoxicillin-clavulanate (amoxicillin concentration, 139.7 μg/ml; clavulanate concentration, 11.6 μg/ml).
At 12 h, a bactericidal effect equivalent to a 5-log10 reduction was again obtained with the 1:2 dilution (Fig. 3). That dilution is equivalent to trough concentrations of 0.2 μg of amoxicillin per ml and <0.01 μg of clavulanate per ml. A bactericidal response was observed up to a dilution of 1:8 (amoxicillin concentration, 0.05 μg/ml; clavulanate concentration, <0.01 μg/ml).
FIG. 3.
Kinetic killing curves for the LLP E. coli strain exposed to serial dilutions of serum (1:2 to 1:32) sampled 12 h after i.v. infusion of 1.1 g of amoxicillin-clavulanate (amoxicillin concentration, 0.4 μg/ml; clavulanate concentration, <0.01 μg/ml).
The bactericidal potencies of the dilutions of serum obtained at the various time points in the pharmacokinetic study are summarized in Table 3.
TABLE 3.
Ex vivo bactericidal activity of amoxicillin-clavulanate at 1.1 g against the four E. coli strains tested
| Log10 decrease in bacterial counta | Corresponding dilution | All samples from the following times or time intervals (h) from the pharmacokinetic study |
|---|---|---|
| LLP | ||
| 5 | 1:2 | 0-12 |
| 5 | 1:2 and 1:4 | 0-5 |
| 5 | 1:2 to 1:16 | 0.5 |
| 3 | 1:2 to 1:8 | 0-12 |
| 3 | 1:32 | 0.5-1 |
| ILP 425 | ||
| 5 | 1:2 | 0.5, 0.75, 1 |
| 5 | 1:2 to 1:4 | 0.5, 0.75 |
| 4 | 1:2 to 1:8 | 0.5 |
| 3 | 1:2 | 1.5 |
| 2 | 1:4 | 1 |
| 1 | 1:2 | 2 |
| ILP 8425 | ||
| 5 | 1:2 | 0.5-2 |
| 5 | 1:2 to 1:4 | 0.5, 0.75 |
| 5 | 1:2 to 1:8 | 0.75 |
| 3 | 1:2 to 1:8 | 0.75 |
| 2 | 1:2 to 1:16 | 0.5 |
| HLP | ||
| 5 | 1:2 | 0.5, 0.75, 1 |
| 5 | 1:2 to 1:4 | 0.5, 0.75 |
| 3 | 1:8 | 0.5 |
| 2 | 1:2 | 1.5 |
Decrease from the starting inoculum.
By using the plots of bactericidal activity, ISBs were calculated for a 3-h period and then a 6-h period. For the LLP strain, Fig. 4A shows ISB as a function of the amoxicillin concentration after 6 h of exposure. Despite divergences in ISB inherent to the natural variability, it should be observed that, overall, the ISBs were constant (about 9%), irrespective of the amoxicillin concentration. The ISBs determined after 3 h of exposure (data not shown) were close to 15%. The lower ISBs after 6 h of exposure and the low value of the regression line slope (y = −4.72x + 15.31) confirm the time-dependent pharmacodynamics of amoxicillin (in the presence of clavulanate) with respect to LLP E. coli strains
Strain ILP 8425.
At 0.5 h the 1:2, 1:4, and 1:8 dilutions induced 5-log10 reductions in bacterial counts after 6 h of exposure. A 5-log10 reduction in the bacterial count was again obtained with the 1:2 dilution at 2 h (Table 3). The amoxicillin concentration was 4.4 μg/ml, and the clavulanate concentration was 1.5 μg/ml.
The ISBs determined from the set of bactericidal plots after 6 h of exposure are shown in Fig. 4B. At a concentration of 10 μg/ml, the ISB was approximately 10%. However, at concentrations below 10 μg/ml there was a trend toward increasing ISBs.
Strain ILP 425.
Very significant bactericidal potencies were obtained with 1:8 dilutions of the peak concentrations of the drugs in serum after up to 6 h of exposure (Table 3), with the equivalent of 5-log10 reductions for the 1:2 and 1:4 dilutions and a 4-log10 reduction for the 1:8 dilution. The last serum dilution enabling marked (3-log10) ex vivo bactericidal potency was obtained at 1.5 h (Table 3), with the amoxicillin concentration equivalent to 8.3 μg/ml and the clavulanate concentration equivalent to 2.1 μg/ml.
The resulting ISBs were approximately 40% after 3 h of exposure (data not shown) and 10% after 6 h of exposure to amoxicillin at concentrations of approximately 20 μg/ml (Fig. 4C). At concentrations below 20 μg/ml following 6 h of exposure, the ISBs were generally higher (20 to 40%).
HLP strain.
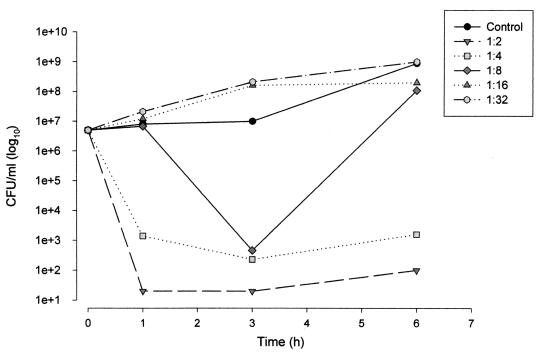
At the time of the peak concentration of drug in serum (0.5 h), a 5-log10 reduction in bacterial counts was obtained after 3 h of exposure to serum diluted 1:2 and 1:4 (Fig. 5). The reduction in the bacterial inoculum was followed by a fast renewal of growth of 2 log10 after 6 h of exposure. The 1:16 dilution induced a bacteriostatic effect. The serum sample obtained at the 1.5-h time point in the pharmacokinetic study was the last one with a bactericidal effect against the strain in question (2 log10), with the amoxicillin trough concentration equivalent to 8.3 μg/ml and the clavulanate trough concentration equivalent to 2.1 μg/ml (Table 3).
FIG. 5.
Kinetic killing curves for the HLP E. coli strain exposed to serial dilutions of serum (1:2 to 1:32) sampled 0.5 h after i.v. infusion of 1.1 g of amoxicillin-clavulanate (amoxicillin concentration, 139.7 μg/ml; clavulanate concentration, 11.6 μg/ml).
At a concentration of 20 μg/ml or greater, the ISB was approximately 9%, irrespective of the amoxicillin concentration. However, at lower concentrations there was once again a general trend toward an increased ISB as the amoxicillin concentration decreased. The slope of the regression line was −35.04 (Fig. 4D).
DISCUSSION
The first part of the study consisted of an investigation of the pharmacokinetics of amoxicillin-clavulanate in a Yucatan miniature pig model. Amoxicillin-clavulanate have similar kinetic behaviors in the Yucatan miniature pig and humans, and the kinetics of the 1.1-g dose in the miniature pig closely approximating those in humans following the administration of 2.2 g i.v. The time at which the serum amoxicillin concentrations were greater than the amoxicillin-clavulanate MIC for a strain, like the LLP strain, for which the MIC was 4 μg/ml was equivalent to 37.5% of the 8-h period separating the two drug intake times (Table 4).
TABLE 4.
Hypothetical times at which the amoxicillin-clavulanate concentrations were greater than the MICs for E. coli strains from pigs (experimental data) and humansa
| MIC (μg/ml) | % Time concn is greater than MICb
|
|
|---|---|---|
| Miniature pig | Human | |
| 0.1 | 100 | 100 |
| 0.5 | 100 | 100 |
| 1.0 | 62.5 | 100 |
| 2.0 | 50 | 87.5 |
The data for humans are from Staniforth et al. (21).
Percentage of the 8-h period separating two intakes at which the serum amoxicillin concentrations were greater than the amoxicillin-clavulanate MIC.
The prior validation of the model enabled us to use it to study the ex vivo antibacterial potency of amoxicillin-clavulanate against β-lactamase-producing strains of E. coli.
In pharmacodynamic terms, the ISB data present the advantage of allowing evaluation of the overall bactericidal activity over a determined period of time. The value of the slope obtained by correlating the ISB and the in vivo concentrations of amoxicillin supports the time-dependent behavior of amoxicillin-clavulanate. These ISBs correlated with the amoxicillin concentrations independently of the clavulante concentrations. However, for the more resistant strains there appears to be a trend toward a concentration dependence for amoxicillin concentrations at or below the MIC. It is possible that this concentration-dependent effect may reflect the limited clavulanate concentrations used. However, in vitro time-kill studies have demonstrated an increased extent and rate of bacterial killing as the β-lactam concentrations are increased up to one to two times their MICs (5). Thus, for amoxicillin concentrations at or near the MIC there is the potential for mixed (both concentration and time-dependent) pharmacodynamic behavior. This has also previously been observed in an in vitro-ex vivo study of the potency of amoxicillin against Streptococcus pneumoniae (14). These findings confirm the importance of achieving concentrations that result in a proportion of the time above the MIC of about 40%.
For the LLP strain, reductions in bacterial numbers as large as 5 log10 were obtained with some dilutions of the test sera in which the drug concentrations were equivalent to trough amoxicillin and clavulanate concentrations and were markedly lower than the MICs and MBCs. This marked bactericidal potency may be related to various factors. First, it is possible that the intrinsic bactericidal activity of the serum may have an effect, but antibiotic-free pig serum was not seen to have bactericidal activity against any of the E. coli strains tested. Moreover, no additive effect of the serum was observed when the MIC was determined with 50% serum (data not shown), regardless of the strain used. To study the addictive effect of pig serum on the bactericidal activity during the MIC determination experiments, bacterial counts were determined after 2 h of contact with antibiotics. To take into account the dilution of broth medium by 50% serum, a control was achieved by use of 50% NaCl (0.9%). No increase in bactericidal activity was measured in 50% pig serum compared with that measured for the control.
Second, it is probable that the MICs and MBCs, measured at the end point by dilution in broth medium, are not equivalent to the real potencies of penicillins against the strain and are, as a consequence, overestimated. Indeed, the MICs and MBCs measure a composite end point of killing and regrowth. The release of β-lactamases may also render the antibiotic ineffective by hydrolytic cleavage of amoxicillin. This may not be a true reflection of the clinical situation, in which dosing would occur every 6 to 8 h. This shows once again the difficulty of establishing a reliable resistance level for this type of β-lactamase-producing strain by the method used (16, 19).
Rapid resumption of growth of the bacterial inoculum was observed following the bactericidal action of the serum on the HLP strain, a high-level β-lactamase producer. The existence of a threshold clavulanate concentration at which the serum no longer had bactericidal activity ex vivo is also of interest. For the HLP and ILP 425 strains, the threshold concentration was about 1.5 to 2 μg/ml, which justifies the use of 2 μg of clavulanate per ml for determination of MICs and MBCs. Clavulanate concentrations therefore seem to be a limiting factor with respect to the bactericidal potency of amoxicillin-clavulanate, and this is probably related to the high levels of β-lactamase produced by these strains.
At the end of the infusion, the high concentrations of β-lactamase inhibitor were able to restore the antibacterial potencies of the β-lactam, even against strains with high-level β-lactamase production. This was observed ex vivo in the first few hours following the start of infusion, when a 5-log10 reduction in the bacterial inoculum was obtained, irrespective of the strain tested (Table 3). The ex vivo bactericidal potency studies were conducted independently of each other, over time, and do not take into account the reduction in the bacterial inoculum. Reguera et al. (19) have shown the correlation that exists between the density of the bacterial inoculum and the efficacy of amoxicillin-clavulanate. Thus, the reduction in bacterial density is accompanied by a reduction in the level of β-lactamase production, which, in vivo, would prolong the bactericidal activity of amoxicillin-clavulanate to time points beyond those obtained ex vivo.
In addition, clavulanate has been reported to exert a post-β-lactamase inhibitor effect against strain 425 (V. Murbach, S. Bronner, L. Linger, R. Dillenseger, W. Sougakoff, N. Dhoyen, H. Monteil, and F. Jehl, Abstr. 19th Interdisc. Meet. Anti-Infect. Chemother., abstr. 97/P1, 1999) and other β-lactamase-producing strains (22). This effect would not have been seen in the present studies since the bacteria were freshly exposed to the sera. The delay in the resumption of growth observed following preexposure to amoxicillin-clavulanate may reach several hours and, in vivo, should increase the duration of the efficacy of amoxicillin-clavulanate against β-lactamase-producing strains, despite the rapid fall in serum clavulanate concentrations.
In conclusion, this study showed that amoxicillin-clavulanate, at concentrations similar to those seen in human plasma following administration of 2.2 g i.v., has bactericidal potential against LLP and ILP E. coli strains. This is in contrast to what is suggested by the MICs and MBCs. The marked reduction in the inoculum over the first few hours, when the antibiotic and inhibitor concentrations are high, and the post-β-lactamase inhibitor effect should further enhance that potency.
This requires demonstration in a miniature pig experimental infection model. The study is ongoing.
REFERENCES
- 1.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavalier, A., D. Levêque, J. D. Peter, J. Salmon, H. Elkhaïli, Y. Salmon, P. Nobelis, J. Geisert, H. Monteil, and F. Jehl. 1997. Pharmacokinetic interaction between itraconazole and ceftriaxone in Yucatan miniature pigs. Antimicrob. Agents Chemother. 41:2029-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaïbi, E. B., D. Sirot, G. Paul, and R. Labia. 1999. Inhibitor-resistant TEM β-lactamases: phenotypic, genetic and biochemical characteristics. J. Antimicrob. Chemother. 43:447-458. [DOI] [PubMed] [Google Scholar]
- 4.Courvalin, P., F. Goldstein, and A. Philippon. 1985. Détermination de la concentration minimale inhibitrice et de la concentration minimale bactéricide en milieu liquide, p. 191-192. In L'Antibiogramme. MPC-Videom Éditeur, Paris, France.
- 5.Craig, W. A., and S. C. Ebert. 1991. Killing and regrowth of bacteria in vitro: a review. Scand. J. Infect. Dis. 74(Suppl.):63-70. [PubMed] [Google Scholar]
- 6.Elkhaïli, H., D. Levêque, J. D. Peter, J. Salmon, Y. Salmon, G. Kaltenbach, A. Cavalier, L. Linger, D. Pompei, H. Monteil, and F. Jehl. 1996. Validation du modèle de microporc Yucatan pour les études pharmacocinétiques du ceftriaxone, céfépime, cefpirome et méropénème. Med. Mal. Infect. 26:599-604. [Google Scholar]
- 7.Elkhaïli, H., D. Levêque, J. D. Peter, Y. Salmon, J. Salmon, D. Pompei, H. Monteil, and F. Jehl. 1997. Pharmacokinetics of three new β-lactams in the Yucatan micropig model administrated by intravenous bolus injection and continuous infusion. Int. J. Antimicrob. Agents 8:135-141. [DOI] [PubMed] [Google Scholar]
- 8.Elkhaïli, H., D. Pompei, J. D. Peter, L. Linger, J. Salmon, D. Levêque, S. Niedergang, Y. Salmon, R. C. Thierry, H. Monteil, and F. Jehl. 1997. Pharmacocinétique de l'amikacine in vivo et sa pharmacodynamie en association avec le céfépime, cefpirome et méropénème dans un modèle in vitro/ex vivo de microporc. Path. Biol. 45:347-356. [PubMed] [Google Scholar]
- 9.Elkhaïli, H., Y. Salmon, J. Salmon, J. D. Peter, D. Levêque, D. Pompei, O. Meunier, H. Monteil, and F. Jehl. 1997. Interest of use of miniature pig in pharmacokinetic studies. J. Pharm. Clin. 16:219-223. [Google Scholar]
- 10.Garraffo, R., H. B. Drugeon, P. Dellamonica, E. Bernard, and P. Lapalus. 1990. Determination of optimal dosage regimen for amikacin in healthy volunteers by study of pharmacokinetics and bactericidal activity. Antimicrob. Agents Chemother. 34:614-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibaldi, M., and D. Perrier. 1982. Drug and pharmaceutical sciences: pharmacokinetics, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 12.Jehl, F. 1993. Acide clavulanique: concentrations efficaces, méthodologies analytiques et pénétration tissulaire. Med. Mal. Infect. 23:1-11. [Google Scholar]
- 13.Jehl, F., C. Gallion, and H. Monteil. 1990. High performance liquid chromatography of antibiotics. J. Chromatogr. 531:509-548. [DOI] [PubMed] [Google Scholar]
- 14.Jehl, F., N. Kamili, H. Elkhaïli, and H. Monteil. 1997. Pharmacodynamie in vitro de l'amoxicilline et bactéricidie ex vivo après 1 g per os sur S. pneumoniae résistants à la pénicilline. Med. Mal. Infect. 27:S45-S57. [Google Scholar]
- 15.Jehl, F., H. Monteil, and J. M. Brogard. 1987. Détermination directe de l'acide clavulanique dans les liquides biologiques par HPLC. Path. Biol. 35:702-706. [PubMed] [Google Scholar]
- 16.O'Shaughnessy, E. M., A. F. Fahle, and F. G. Witebski. 1997. Correlation of in vitro susceptibility results for amoxicillin-clavulanate and ampicillin-sulbactam tested against Escherichia coli. J. Clin. Microbiol. 35:1902-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panepinto, L., and R. W. Phillips. 1986. The Yucatan miniature pig: characterization and utilization in biomedical research. Lab. Anim. Sci. 36:344-347. [PubMed] [Google Scholar]
- 18.Panepinto, L. M., R. W. Phillips, S. Nordern, P. C. Pryor, and R. Cox. 1983. A comfortable, minimum stress method of restraint for Yucatan miniature swine. Lab. Anim. Sci. 33:95-97. [PubMed] [Google Scholar]
- 19.Reguera, J. A., F. Baquero, J. C. Pérez-Díaz, and J. L. Martinez. 1991. Factors determining resistance to β-lactam combined with β-lactamase inhibitors in Escherichia coli. J. Antimicrob. Chemother. 27:569-575. [DOI] [PubMed] [Google Scholar]
- 20.Salmon, J., D. Levêque, J. D. Peter, A. Cavalier, H. Elkhaïli, Y. Salmon, G. Kaltenbach, H. Monteil, and F. Jehl. 1996. Modèle de porc miniature cathétérisé pour les études pharmacocinétiques et pharmacodynamiques des agents antiinfectieux. Path. Biol. 44:375-378. [PubMed] [Google Scholar]
- 21.Staniforth, D. H., D. Jackson, R. Horton, and B. Davis. 1984. Parenteral Augmentin: pharmacokinetics. Int. J. Clin. Pharmacol. Ther. Toxicol. 22:430-434. [PubMed] [Google Scholar]
- 22.Thorburn, C. E., S. J. Molesworth, R. Sutherland, and S. Rittenhouse. 1996. Postantibiotic and post-β-lactamase inhibitor effects of amoxicillin plus clavulanate. Antimicrob. Agents Chemother. 40:2796-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou, X. Y., F. Bordon, D. Sirot, M. D. Kitzis, and L. Gutmann. 1994. Emergence of clinical isolates of Escherichia coli producing TEM-1 derivatives or an OXA-1 β-lactamase conferring resistance to β-lactamase inhibitors. Antimicrob. Agents Chemother. 38:1085-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]