Abstract
Molecular evolution of multiresistance in nontyphoid Salmonella spp. was investigated with 155 isolates obtained in Argentina from 1984 to 1998. In 74 isolates obtained from 1984 to 1988 resistance was associated with the presence of Tn3, Tn9, class I (In0) and II (Tn7) integrons, and the aac(3)-IIa gene. Extended-spectrum cephalosporin (ESC) resistance in Salmonella spp. emerged in 1989, and 81 isolates resistant to at least one ESC and one aminoglycoside were collected thereafter. Among these, two patterns of antimicrobial resistance mechanisms were found: from 1989 to 1992, resistance was related to the spreading of Tn1331 and blaCTX-M-2, in addition to the persistence of In0 and Tn7. From 1993 to 1998, several integrons were added to the first pattern and three integron groups (IG), namely, IG1 (38% of the isolates), IG2 (51%), and IG3 (11%), were identified. At least two β-lactamase genes were detected in 65% of the isolates (after 1989) by PCR analysis. Furthermore, five β-lactamase genes, blaCTX-M-2, blaOXA-9, blaOXA-2, blaTEM-1, and blaPER-2, were found in two isolates. The blaCTX-M-2 gene was found in several complex sulI-type integrons with different rearrays within the variable region of class I integrons, suggesting evolution of these integrons in nontyphoid Salmonella. In conclusion, progressive acquisition and accumulation of plasmid-mediated resistance determinants occurred from 1984 to 1998 in nontyphoid Salmonella isolates of the most prevalent serovars from Argentina. It is suggested that antimicrobial resistance mechanisms in these bacteria may have been the consequence of plasmid exchange between Salmonella enterica serovar Typhimurium and Escherichia coli or Shigella flexneri and/or spreading of mobile elements from the nosocomial environment.
Nosocomial nontyphoid Salmonella infections have been reported in recent years from many geographic areas, including countries with high public health and hygiene standards (13, 19, 26). Multiresistant Salmonella isolates of different serovars are increasingly common, appear with variable geographical incidence, and have become an issue of worldwide concern (10, 30). Currently, several industrialized countries have ongoing programs on surveillance of multiresistance in zoonotic bacteria, including Salmonella. Serovars of this genus, other than Salmonella enterica serovar Typhi, are major agents of gastroenteritis and can also cause systemic infections in animals and humans (39, 41). Despite the fact that Salmonella spp. are not typical members of the hospital microflora, several outbreaks due to multiresistant isolates have been reported in hospitals from Argentina, as well as in other countries (25, 26, 38). Although antibiotic therapy is not usually recommended for treatment of patients with Salmonella gastroenteritis, invasive complications such as meningitis, sepsis, and bacteremia require it. Combinations of β-lactams and an aminoglycoside are widely used in Argentina in the treatment of neonatal and pediatric nosocomial infections due to Salmonella spp. It has been suggested that extended-spectrum β-lactamases (ESBL) associated with plasmids are responsible for the extended-spectrum cephalosporin (ESC) resistance. In a national surveillance study for ceftriaxone-resistant Salmonella infections in the United States, blaCMY-2 has been found to be the most prevalent β-lactamase gene causing ESC resistance (17). Although a few ESBL in Salmonella spp. causing short-term nosocomial outbreaks have been reported (5, 6, 18, 27, 39), only PER-1 was recognized in multiple-antibiotic-resistant S. enterica serovar Typhimurium strains isolated over a 28-month period from the nosocomial environment in Turkey (38).
From 1969 to 1985 the most prevalent Salmonella serovar in hospitals from different cities of Argentina was Salmonella serovar Typhimurium, followed by S. enterica serovar Oranienburg. S. enterica serovar Enteritidis emerged in 1986 and since 1987 has been the most frequent serovar, with the exception of 1991 and 1992, when the most prevalent serovars were, respectively, Salmonella serovar Typhimurium and S. enterica serovar Infantis. Salmonella serovar Enteritidis has been mainly involved in food-borne outbreaks. Before 1989, resistant nosocomial Salmonella isolates exhibited resistance to aminoglycosides, ampicillin, chloramphenicol, and/or sulfonamides. In 1989 resistance to ESC emerged in pediatric hospitals of Argentina, reaching levels of combined aminoglycoside and ESC resistance of over 40% (26, 33). Resistance to ESC was detected first in Salmonella serovar Typhimurium and later in Salmonella serovar Infantis and Salmonella serovar Agona; it was rarely detected in Salmonella serovar Enteritidis. No resistance to ciprofloxacin was detected in Salmonella isolates from Argentina.
This study was designed to (i) determine the resistance genes involved in five multiresistant serovars of Salmonella spp. strains isolated from nosocomial infections in Argentina, (ii) evaluate the evolution of the mechanisms involved in the spreading of antimicrobial resistance in a significant number of multiresistant nontyphoid Salmonella spp. isolated between 1984 and 1998, and (iii) scrutinize those resistance mechanisms found in Salmonella spp. in the gram-negative population of the same hospital environment.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A total of 155 isolates were utilized in this study (Table 1). Isolates were obtained from stools (97%), blood (2%), and urine (1%). All isolates were biochemically (API 20E strip; BioMérieux) and serologically identified according to the standard international scheme for Salmonella serotyping (32) at the reference laboratory INEI-ANLIS “Dr. Carlos G. Malbrán.” Criteria for strain selection were based upon the emergence of antimicrobial resistance, and two resistance phenotypes were selected. Group R1, from the period 1984 to 1988, included 74 isolates resistant to ampicillin and gentamicin. Group R2 (81 isolates) included Salmonella spp. resistant to at least one ESC and at least one aminoglycoside; these were obtained from 1989, when ESC resistance in the genus Salmonella emerged in Argentina. To determine the putative transference of resistance determinants into Salmonella from other isolates in the same nosocomial environment, the presence of plasmids and antimicrobial resistance mechanisms in Enterobacteriaceae isolates were investigated. Enterobacteriaceae isolates were obtained from the same hospitals and within the same periods as the Salmonella isolates, and a number of them were obtained from patients that were coinfected with Salmonella spp. During the course of the outbreak in H1 (Table 1), 15 patients were concomitantly infected with Shigella flexneri and/or Escherichia coli resistant to at least two antimicrobial agents. These two species were recovered mainly from stools and urine, respectively. We investigated one E. coli isolate and one S. flexneri isolate from the outbreak. From 1989 to 1998, 55 seemingly epidemiologically unrelated E. coli (n = 5), Klebsiella pneumoniae (n = 17), Serratia marcescens (n = 3), Citrobacter freundii (n = 4), Enterobacter cloacae (n = 6), Enterobacter aerogenes (n = 5), and Proteus mirabilis (n = 20) isolates were selected. These isolates were resistant to ESC and to at least one aminoglycoside, and they were isolated from nosocomial infections from the same hospitals where the Salmonella sp. strains had been collected. Cultures were routinely performed at 37°C in brain heart infusion agar (Difco Laboratories, Detroit, Mich.) supplemented with ampicillin (50 μg/ml) or cefotaxime (16 μg/ml), as required.
TABLE 1.
Characteristics of nontyphoid Salmonella sp. isolates
| Salmonella serovar | No. of isolates | Hospitala | Yr of isolation | Type of infectionb |
|---|---|---|---|---|
| Typhimurium | 65 | H1 | 1984 | O |
| Typhimurium | 5 | H1 | 1985 | NI |
| Typhimurium | 4 | H2 | 1987 | NI |
| Typhimurium | 8 | H2 | 1990 | O |
| Agona | 26 | H2 | 1990 | O |
| Infantis | 4 | H2 | 1990 | O |
| Oranienburg | 12 | H2 | 1991 | O |
| Enteritidis | 10 | H2 | 1991 | O |
| Typhimurium | 5 | H3 | 1991 | NI |
| Typhimurium | 4 | H3 | 1993 | NI |
| Infantis | 2 | H4 | 1994 | NI |
| Typhimurium | 3 | H4 | 1995 | NI |
| Agona | 2 | H4 | 1995 | NI |
| Infantis | 4 | H5 | 1996 | NI |
| Typhimurium | 1 | H6 | 1998 | NI |
H1, hospital in La Plata, 70 km from Buenos Aires; H2 to H6, hospitals in Buenos Aires.
O, outbreak; NI, sporadic nosocomial infection (seemingly epidemiologically unrelated nontyphoidal sp. isolates).
Antimicrobial susceptibility testing.
Tests for susceptibility to ampicillin, cefotaxime, ceftazidime, amikacin, gentamicin, chloramphenicol, trimethoprim-sulfamethoxazole, imipenem, and cefoxitin were performed by using the agar diffusion method according to the guidelines of the National Committee for Clinical Laboratory Standards (29). Antibiotics were purchased from Sigma Chemical Co. (St. Louis, Mo.). MICs for the Salmonella sp. strains from the R2 group were determined on Mueller-Hinton agar (Difco) plates containing serial twofold dilutions of the following antimicrobial agents: cefotaxime, ceftazidime, amikacin, and gentamicin (29). An inoculum of 104 bacteria per spot was deposited with a Steer inoculator.
Conjugation and plasmid analysis.
Nalidixic acid-resistant E. coli C600 was used as the recipient in conjugation experiments. Conjugation was performed in brain heart infusion media for 4 h at 37°C, and transconjugants were selected on Mueller-Hinton agar containing ampicillin (50 μg/ml) or cefotaxime (4 μg/ml) plus nalidixic acid (100 μg/ml).
DNA techniques.
Total and plasmidic DNA was prepared as described before (16, 34). Plasmidic DNA was digested with different restriction enzymes, and the fragments were separated in horizontal gels of 0.8 to 1.0% (wt/vol) agarose dissolved in 0.4 M Tris-acetate-0.01 M EDTA. PCR amplifications were carried out in 100-μl volumes containing 10 ng of DNA, 10 μl of 10× PCR buffer, 10 μl of 10× deoxynucleoside triphosphate mixture (2 mM [each] dATP, dCTP, dGTP, and dTTP), 10 μl of each primer stock solution (2.5 pmol of each primer per μl), and 60 μl of sterile distilled water. Each reaction mixture was covered with 75 μl of mineral oil. Taq DNA polymerase (Promega) was added (1 μl of the 3-U/μl diluted solution) after 12 min at 94°C (hot-start method). To amplify the DNA in the thermocycler (Perkin-Elmer Cetus, Emeryville, Calif.), a three-step profile was utilized (23). Primers for PCRs were specific for the nucleotide sequences exhibited in Table 2. PCR mapping of integrons, transposons, and unusual class I integrons was done as follows (see also Table 2). (i) Class I and II integron mapping was performed with primers for the 5′ and 3′ conserved segments. To determine the gene order in the variable region, we used primers for sequences located at the ends of the inserted resistance gene cassettes in combination with those specific for other gene cassettes, as well as with those for the conserved segments. (ii) Tn3 mapping was performed with specific primers for the transposase and the blaTEM gene. Tn1331 mapping was performed with primers for the transposase (which is identical to that of Tn3) and for aac(6′)-Ib. Tn9 mapping was performed with specific primers for the cat gene. (iii) The mapping of the unusual class I integrons was performed with specific primers for ORF513 and the blaCTX-M-2 gene. To identify the class I integron variable region, we used primers located at the ends of the last inserted cassette in the variable region and a primer for the blaCTX-M-2 gene which rendered amplicons of approximately 4,000 bp. E. coli and S. flexneri plasmid DNA digested with EcoRI, BamHI, and HindIII was transferred to nylon filters by Southern blotting and hybridized with plasmid DNA of Salmonella serovar Typhimurium from the R1 group digested with HindIII as described elsewhere (34). Colony hybridization was performed as described previously (34). Kodak X-Omat AR film was used for autoradiography. Several PCR products, amplified with cloned Pfu DNA polymerase (Stratagene, La Jolla, Calif.), were sequenced after purifying the DNA by using the QIAquick kit according to the manufacturer's directions (Qiagen Inc., Studio City, Calif.). Sequencing was performed on both DNA strands with an ABI 373 sequencer. Internal oligonucleotide primers were used where necessary to ensure that both strands were sequenced. Several blaCTX-M-2- like, blaPER-2-like, and blaTEM-1-like PCR products that were sequenced corresponded to blaCTX-M-2, blaPER-2, and blaTEM-1 genes, respectively; the remaining β-lactamase-encoding PCR products were subsequently named blaCTX-M-2-like, blaPER-2-like, and blaTEM-1-like, respectively. The nucleotide sequences were analyzed with the Genetics Computer Group software.
TABLE 2.
Primers used for PCRs
| Amplified DNAe | Primer | Oligonucleotide sequence (5′-3′) | Accession no. and reference |
|---|---|---|---|
| Class I integrons | |||
| intiI | IntiIf | TTC GAA TGT CGT AAC CGC | M73819g |
| IntiIr | CGA GGC ATA GAC TGT AC | M73819g | |
| Sulpro3 | GCC TGA CGA TGC GTG GA | M73819 (23) | |
| 3′ conserved segment | 3′-CS | AAG CAG ACT TGA CCT GA | M73819 (23) |
| Class II integrons | |||
| intiII | IntiIIf | GCA AAT GAA GTG CAA CGC | AF318072g |
| IntiIIr | ACA CGC TTG CTA ACG ATG | AF318072g | |
| Class III integrons | |||
| intiIII | IntiIIIf | AGG TGC CTC CGG CAG CG | AF4162297g |
| IntiIIIr | TGT CTG TGG ACC CAC AA | AF4162297g | |
| Tn1331 | |||
| tnpR | TnpR | AAG TTC ATC GGG TTC GC | AF479774g |
| aac(6′)-Ib | aac6-Ibr | TGT GAC GGA ATC GTT GC | AF227505 (23) |
| Tn3 and blaTEM-like | |||
| tnpR | TnpR | AAG TTC ATC GGG TTC GC | AF479774g |
| blaTEM | 201r | TCG CCG CAT ACA CTA TTC T | AF516720g |
| ORF513 | Orf513fd | GCG AAC ACT GCG GCG GTC AC | L06418 (2) |
| Orf513rd | CTG AGG GTG TGA GCG AG | L06418g | |
| Chloramphenicol Rf gene (Tn9) | Catf | GGT GAG CTG GTG ATA TGG | V00622g |
| Catr | GGG ATT GGC TGA GAC GA | V00622g | |
| β-Lactam R genes | |||
| blaOXA-2 (OXA-15, -32, -34) | Oxa-2fb | GAA GAA ACG CTA CTC GC | AF315351g |
| Oxa-2ra | TAC CCA CCA ACC CAT AC | AF315351g | |
| blaOXA-9 | Oxa-9fbb | GAA CAC CAA CAT ATG CA | AF034958g |
| Oxa9ra | GGG ACA ATA ACG GCA AG | AF034958g | |
| blaOXA-11 (OXA-13, -14, -16, -17, -19, -28, -35) | Oxa 11f | ACT CAG TTC CCA CAC CA | AF300984g |
| Oxa11r | TCC CCA ACG CAA TTA TC | AF300984g | |
| blaCTX-M-2 (KLUA-1, KLUA-2, CTX-M-4, TOHO-1) | Ctx-M2f | ATG ACT CAG AGC ATT CGC | AF286192g |
| Ctx-m2rd | TCA CTT TAT CGG GAC CAC | AF286192g | |
| blaPER-2 | Per-2f | CGC TTC TGC TCT GCT GAT | X93314g |
| Per2r | GGC AGC TTC TTT AAC GCC | X93314g | |
| blaFOX-2 | Foxf | AGT TCC CTG ATG AGG TG | Y10282g |
| Foxr | GAA TAG CCG TAG GCA TAG | Y10282g | |
| blaSHV (SHV-1, -2, -5-8, -11, -12, -14, -18, -26-29, -33-35, -38) | Shvf | ATT ACC ATG AGC GAT AAC A | AF462394g |
| shvr | GTA TCC CGC AGA TAA ATC A | AF462394g | |
| Aminoglycoside R genes | |||
| aac(6′)-Ib | Aac6Ibra | TGT GAC GGA ATC GTT GC | AF227505 (23) |
| Aac6Ibfb,c | AAA CAC GCC AGG CAT TC | AF227505g | |
| aac(6′)-Iq | Aac6Iqa | GAC TTT CCC AAT ACC CC | AF047556g |
| aac(3)-IIa | AacC2f | CGC TAA ACT CCG TTA CC | M62833g |
| AacC2r | TAG CAC TGA GCA AAG CC | M62833g | |
| ant(2′′)-Ia | AadBfa | CTA TGC CGA TGA AGT ACC | AF453998 (23) |
| AadBrb,c | AGA CCT CAA CCT TTT CC | AF453998g | |
| ant(3′′)-Ia | AadA1ra | TGC ATG ACG CCA ACT AC | AF327064 (23) |
| AadA1fb,c | CGC AGA TCA GTT GGA AG | AF327064g | |
| Trimethoprim R gene dfrAI | dfrAIra | AGC TGT TCA CCT TTG GC | AF455254 (23) |
| dfrAIfb | CCT GAA ATC CCC AGC AA | AF455254g | |
| orfD | OrfDra | CAT TCT GCG GTC GGC TT | U13880g |
| OrfDfb,c | CAT TCT GCG GTC GGC TT | U13880g |
Primer used to perform PCR mapping of class I integrons in combination with the Sulpro3 primer from the intiI gene. The PCR amplicons differ in expected size depending on their locations in the class I integron variable region.
Primer used to perform PCR mapping of class I integrons in combination with the 3′-CS primer from the 3′ conserved segment. The PCR amplicons differ in expected size depending on their locations in the class I integron variable region.
Primer used to perform PCR mapping in combination with the Orf513r primer (PCR amplicons with an average length of approximately 3,000 bp) and with the Ctx-m2r primer (PCR amplicons with an average length of approximately 4,000 bp) to identify the unusual class I integron rearrangements.
Primer used to detect the blaCTX-M-2 gene in the unusual class I integrons.
Designations in parentheses in subscripts of bla genes represent the genes also detected with the corresponding primer combination. The remaining sets of bla primers are specific for the gene or mechanism detected.
R, resistance.
Accession number of the sequence of a primer designed for this study.
RESULTS
Antimicrobial resistance of Salmonella spp.
According to their susceptibility profiles, four phenotypes of group R1 and five phenotypes of group R2 were identified in 155 Salmonella sp. isolates (Tables 3 and 4). All isolates were susceptible to cefoxitin and imipenem, whereas 95% of the isolates were resistant to gentamicin. As shown in Tables 3 to 5, emergence of cefotaxime resistance was observed in the R2 group.
TABLE 3.
Antibiotic resistance phenotypes of nontyphoidal Salmonella serovar Typhimurium isolates in resistance group R1
| Hospitala | Yr of isolation | No. of isolates in resistance subgroupb:
|
|||
|---|---|---|---|---|---|
| R1a | R1b | R1c | R1d | ||
| H1 | 1984 | 10 | 19 | 5 | 27 |
| H1 | 1985 | 2 | 3 | ||
| H2 | 1987 | 4 | 4 | ||
Hospitals are identified in Table 1.
Resistance subgroups are defined as follows: R1a, resistance to ampicillin (AP), gentamicin (GN), and trimethoprim-sulfamethoxazole (SXT); R1b, resistance to AP, chloramphenicol (CM), and GN; R1c, resistance to AP and GN; R1d, resistance to AP, GN, CM, and SXT.
TABLE 4.
Antibiotic resistance phenotypes of nontyphoidal Salmonella sp. isolates in resistance group R2
| Salmonella serovar | No. of isolates in resistance subgroupa:
|
Hospitalb | Yr of isolation | ||||
|---|---|---|---|---|---|---|---|
| R2a | R2b | R2c | R2d | R2e | |||
| Typhimurium | 4 | 4 | H2 | 1990 | |||
| Agona | 7 | 10 | H2 | 1990 | |||
| Infantis | 2 | 1 | 1 | H2 | 1990 | ||
| Oranienburg | 3 | 9 | H2 | 1991 | |||
| Enteriditis | 6 | 3 | 1 | H2 | 1991 | ||
| Typhimurium | 4 | 1 | H3 | 1991 | |||
| Typhimurium | 4 | H3 | 1993 | ||||
| Infantis | 2 | H4 | 1994 | ||||
| Typhimurium | 3 | H4 | 1995 | ||||
| Agona | 1 | 1 | H4 | 1995 | |||
| Infantis | 4 | H5 | 1996 | ||||
| Typhimurium | 1 | H6 | 1998 | ||||
Resistance subgroups are defined as follows: R2a, resistance to cefotaxime (CTX) and gentamicin (GN); R2b, resistance to CTX, amikacin (AK), and GN; R2c, resistance to CTX, ceftazidime (CAZ), AK, and trimethoprim-sulfamethoxazole (SXT); R2d, resistance to CTX, AK, and chloramphenicol (CM); R2e, resistance to CTX, CAZ, GN, CM, and SXT.
Hospitals are identified in Table 1.
TABLE 5.
Levels of susceptibility of Salmonella isolates to β-lactams and aminoglycoside antibiotics
| Antibiotic | MIC (μg/ml)
|
|
|---|---|---|
| Range | 90c | |
| R1 group TEM-1a | ||
| Ampicillin | 64->256 | 128 |
| Cefotaxime | 0.03-0.5 | 0.06 |
| Ceftazidime | 0.06-0.25 | 0.06 |
| Amikacin | 0.5-2 | 1 |
| Gentamicin | 32-128 | 32 |
| R2 group CTX-M-2b | ||
| Ampicillin | >1,024 | >1,024 |
| Cefotaxime | 32-512 | 64 |
| Cefotaxime-clavulanic acid | 0.015-0.03 | 0.03 |
| Ceftazidime | 1-16 | 1 |
| Amikacin | 1-64 | 32 |
| Gentamicin | 32-256 | 64 |
All 74 Salmonella sp. isolates from the R1 group harbored blaTEM-1 according to PCR analysis.
All 81 Salmonella sp. isolates from the R2 group harbored blaCTX-M-2. The blaOXA-9, blaOXA-2, blaTEM-1, and/or blaPER-2-like genes were also tested by PCR analysis.
90, MIC at which 90% of the isolates were inhibited.
Determination of antimicrobial resistance mechanisms other than bla genes.
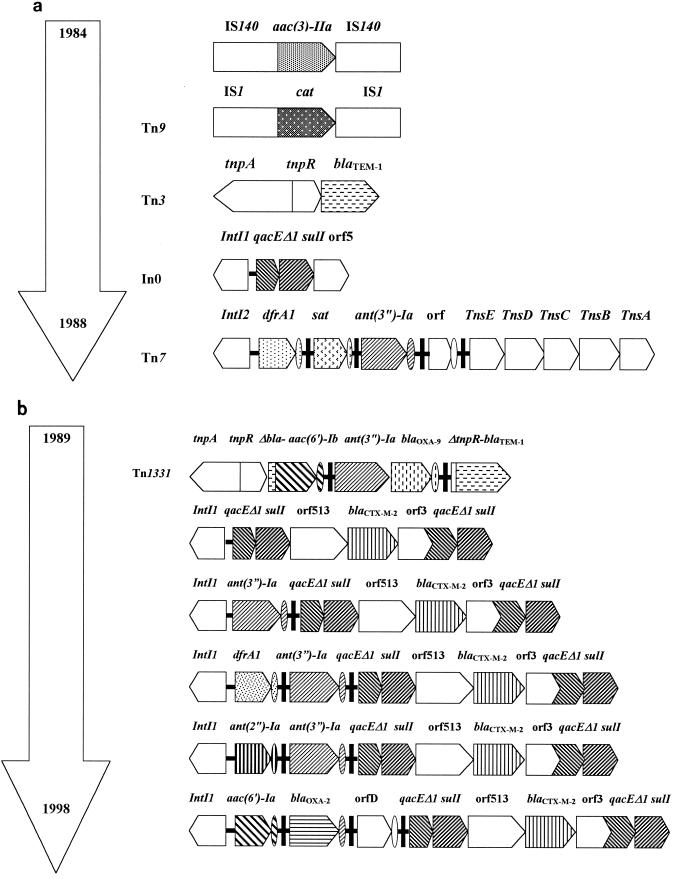
All isolates from the R1 and R2 groups were analyzed by PCR for the presence of Tn7, Tn9, Tn3, Tn1331, class I and II integrons, aac(3)-IIa, and ORF513, previously known as ORF341 (GenBank accession no. L06418). From 1984 to 1988 (group R1) Salmonella serovar Typhimurium isolates harbored Tn3 (blaTEM-1, which confers resistance to ampicillin), Tn9 (cat, which confers resistance to chloramphenicol), aac(3)-IIa (which confers resistance to gentamicin), Tn7 (dfrA1, which confers resistance to trimethoprim), and/or In0 (sulI, which confers resistance to sulfamethoxazole) (Fig. 1a). In the R2 group (1989 to 1998), two patterns of antimicrobial resistance mechanisms were found and all isolates harbored at least one class I integron. The first pattern, from 1989 to 1992, was associated with the emergence of ESC and amikacin resistance by acquisition of blaCTX-M-2 (100% of isolates) and Tn1331 (40% of isolates), respectively. At least one of the integrons, either In0 or In4 [with ant(3")-Ia within the variable region], was harbored by every isolate from this period (1989 to 1992). The second pattern, found between 1993 and 1998, involved blaCTX-M-2, at least two of the same antimicrobial determinants which were found in the R1 group, Tn1331 in 9.5% of the isolates, and several class I integrons conferring resistance to a wide variety of antimicrobial agents. Three integron groups (IG) were harbored by this second pattern. IG1 (38% of isolates) harbored two class I integrons with ant(2")-Ia-ant(3")-Ia and orfD cassettes within the variable region; IG2 (51%) harbored three class I integrons with dfrA1-ant(3")-Ia, aac(6′)-Ib-orfD, and ant(3")-Ia cassettes; IG3 (11%) harbored two class I integrons with aac(6′)-Ib-blaOXA-2-orfD and orfD cassettes.
FIG. 1.
Physical maps of the antimicrobial resistance determinants from 1984 to 1998, showing the evolution and accumulation of antimicrobial resistant determinants in the R1 group (1984 to 1988) (a) and the R2 group (1989 to 1998) (b). Only class I integrons harboring ORF513 are shown. orf3 and qacEΔ1 have been found in In35 as a gene fusion (2).
Tn7 and Tn9 were found in both R1 and R2 group isolates as well as aac(3)-IIa, which was found in 95% of Salmonella sp. isolates. Thirty-eight percent of Salmonella sp. isolates from the R2 group harbored two genes coding for gentamicin resistance, aac(3-IIa) and ant(2")-Ia. Tn1331 (49.5% of R2 isolates), aac(3)-IIa and blaCTX-M-2 genes were found in isolates belonging to all five Salmonella serovars (Table 6). Salmonella serovar Typhimurium, Salmonella serovar Infantis, and Salmonella serovar Agona harbored the three IGs, whereas Salmonella serovar Typhimurium harbored both class I and class II (Tn7) integrons.
TABLE 6.
Detection of antimicrobial resistance determinants in five serovars of genus Salmonella
| Salmonella serovar | Presence of determinant:
|
||||||
|---|---|---|---|---|---|---|---|
| Tn3 | Tn9 | Tn1331 | aac(3)-IIa | blaCTX-M-2 | Class Ia | Class IIb | |
| Typhimurium | + | + | + | + | + | + | + |
| Agona | + | + | + | + | + | + | |
| Enteritidis | + | + | + | + | + | ||
| Oranienburg | + | + | + | + | + | ||
| Infantis | + | + | + | + | + | ||
Class I integrons.
Class II integrons.
Characterization of β-lactamase genes.
blaTEM-1 was found in all Salmonella sp. isolates from group R1 and 65% of group R2 isolates, as shown by PCR and sequencing studies. The blaCTX-M-2-like gene was found in all Salmonella sp. isolates belonging to group R2. The blaPER-2-like gene was found in 2.5% of the isolates, whereas the blaFOX-1-like and the blaSHV-like genes were never found.
The blaOXA-2 and the blaOXA-9 genes were found in 11 and 49.5% of R2 group isolates, respectively, whereas blaOXA-11 was never found. At least two β-lactamases were found in 73% of the R2 group isolates by PCR analysis. Twenty-seven percent possessed the blaCTX-M-2-like gene alone; 47% possessed blaCTX-M-2-like, blaOXA-9, and blaTEM-1-like genes; 15% possessed blaCTX-M-2-like and blaTEM-1-like genes; 8.5% possessed blaCTX-M-2-like and blaOXA-2 genes; and 2.5% possessed blaCTX-M-2-like, blaOXA-9, blaOXA-2, blaTEM-1-like, and blaPER-2-like genes. The blaCTX-M-2 gene was previously found in the unusual class I integrons (2, 15), and in this study it was located at the same position in the genetic structures which characterize this group, involving different arrays of cassettes in the variable regions of the isolates studied [In0 (n = 40), In4 (n = 28), In35 (n = 3), ant(2")-Ia-ant(3")-Ia (n = 8) and dfrA1-ant(3")-Ia (n = 2) within the variable region] by PCR cartography (Fig. 1b). Colony hybridization of the R1 and R2 group isolates was performed by using as a probe ORF513 from the unusual class I integrons, where blaCTX-M-2 and other resistance genes (31, 35, 40) are located. ORF513-related sequences were detected in all R2 group isolates, whereas they were not found in isolates from the R1 group, which did not harbor blaCTX-M-2.
Scrutiny of resistance determinants in other gram-negative Enterobacteriaceae.
To establish the chromosomal or plasmidic location of the genetic determinants for gentamicin, chloramphenicol, trimethoprim-sulfamethoxazole, and ampicillin resistance, conjugation analysis was performed on isolates of the R1 group. Plasmids of 40 kbp, which transferred resistance to ampicillin, gentamicin, and chloramphenicol, were found in five Salmonella serovar Typhimurium isolates, one S. flexneri isolate, and one E. coli isolate. These plasmids were identified as similar, if not identical, by digestion with three DNA endonucleases (EcoRI, HindIII, and BamHI) and by DNA-DNA hybridization analysis using as a probe the 40-kbp Salmonella serovar Typhimurium plasmid DNA digested with HindIII. The antimicrobial determinants blaTEM-1, aac(3)-IIa, and Tn9 were found in the conjugative plasmids of the three species. The same antimicrobial determinants [blaTEM-1, aac(3)-IIa, Tn7, In0, and Tn9] were also found in nine seemingly epidemiologically unrelated Salmonella serovar Typhimurium isolates obtained from H1 and H2 in 1985 and 1987, respectively.
From 1989 to 1998, a total of 55 multiresistant Enterobacteriaceae isolates including E. coli, K. pneumoniae, S. marcescens, C. freundii, E. cloacae, E. aerogenes, and P. mirabilis were analyzed for the presence of blaCTX-M-2, aac(3)-IIa, and class I and II integrons by PCR cartography. The same class I integrons that were described for IG1, IG2, and IG3, as well as Tn7, aac(3)-IIa, and blaCTX-M-2, were found in the above-mentioned bacterial population. However, several other rearrangements in class I and II integrons were also found (data not shown). Conjugation experiments with 10 Salmonella sp. isolates (two of each serovar), 12 P. mirabilis isolates, and 2 K. pneumoniae isolates demonstrated that cefotaxime, gentamicin, and amikacin resistance was carried by conjugative plasmids (data not shown).
DISCUSSION
The findings of the present study on frequency and spreading of class I and II integrons among nontyphoidal Salmonella serotypes permit the following conclusions. (i) Evolution of class I integrons is presumed, since In0 was detected in early isolates dating from 1984, whereas several cassettes inserted within the variable region were found in contemporary isolates. (ii) Accumulation of class I integrons was the mechanism involved in the expression of multiresistance in the five serovars. (iii) Class II integrons were detected in our collection from 1984, while their spreading was limited to one serovar (Salmonella serovar Typhimurium). (iv) The spreading of blaCTX-M-2 was linked to several class I integrons. (v) Although certain class I integrons found in the bacterial population under scrutiny were consistent with others found previously in this genus elsewhere (9, 21, 28, 37), In35 was found only in isolates from Argentina.
Our results clearly showed that, in Argentina, the emergence of resistance to ESC in 1989 in the genus Salmonella was due to acquisition of the blaCTX-M-2 gene. Although several CTX-M-like enzymes from all over the world have been described (http//www.lahey.org/studies/webt/htm), this is the first report that describes a CTX-M-like enzyme in several serovars of nontyphoid Salmonella spp. involved in outbreaks. In addition to our findings on blaCTX-M-2, only very recently blaCTX-M-3 and blaCTX-M-10 were found to be responsible for the prevalence over 10 years as a mechanism for ESC resistance in other gram-negative organisms (8, 12). The blaCTX-M-2-like gene spreading is mediated by conjugative plasmids. In our Enterobacteriaceae population, several other ESBL have been described, such as PER-2, FOX-1, SHV-5, and SHV-2a (4, 20; J. M. Casellas, personal communication). All four enzymes may be considered ceftazidimases rather than cefotaximases (7, 24). PER-1, which possesses 86.4% homology with PER-2, has been found to be responsible for ESC resistance in Salmonella serovar Typhimurium isolates from Turkey (38). Our results, however, demonstrated the high prevalence of CTX-M-2-like enzymes in our Salmonella sp. population, even though PER-like enzymes were also widespread in our geographical area. This event could be related to, among other factors, the widespread use of cefotaxime and ceftriaxone rather than ceftazidime in our hospitals. This is the first report in which ESBL spreading and the epidemiological evolution of class I and II integrons in Salmonella spp. are described. The location of blaCTX-M-2 in the unusual class I integrons and its presence in conjugative plasmids may explain the high dissemination of this ESBL among Salmonella sp. isolates from Argentina.
With regard to multiresistance propagation, on one hand, horizontal acquisition of resistance determinants is usually mediated by plasmids, transposons, or cassettes located in integrons. In fact, conjugative plasmids of the same Inc groups in Enterobacteriaceae isolates before and after medical use of antibiotics have been described (14, 22). Moreover, acquisition of transferable plasmids by Salmonella spp. from other enteric bacteria of the gut flora in the intestinal tract of individual patients has previously been reported (3). On the other hand, dissemination of a multiresistant clone over more than 10 years in Salmonella serovar Typhimurium DT104 has also been described (25). There are at least two hypotheses to explain the fact that the same antimicrobial resistance determinants, blaCTX-M-2, Tn1331, Tn9, aac(3)-IIa and IG1, IG2, or IG3, have been found in the five Salmonella serovars from the present study: (i) the dissemination of a clone with identical resistance determinants for each Salmonella serovar and/or (ii) plasmid spreading and ulterior selection of certain resistance determinants and integrons by the Salmonella genus by independent events, possibly due to the close relationship between the cellular biology of Salmonella spp. and the antimicrobial pressure exerted in pediatric patients in every hospital environment. Although dissemination of resistance determinants through integrons within related plasmids over a long period of time in Salmonella serovar Typhimurium has been described (6), sequential acquisition of integrons, blaCTX-M-2, Tn1331, and Tn9 within plasmids and the ulterior spreading of these plasmids cannot be ruled out. Therefore, it is not unlikely that the strains under scrutiny in this study may have acquired resistance genes from a common nosocomial source. Most probably, the combination of hypotheses i and ii may be the most likely explanation for the emergence of multiresistant group R2.
Although the antimicrobial therapy for pediatric inpatients suffering nosocomial infections due to Salmonella spp. has not been significantly modified from 1989 to the present day, evolution of the antibiotic mechanisms in the R2 group, leading to the accumulation of antimicrobial resistance mechanisms after 1993, has been observed. In isolates obtained from that year to 1998, several antimicrobial determinants conferring resistance to the same antimicrobial agent were observed. This feature was seen for ampicillin (blaCTX-M-2, blaOXA-9, blaOXA-2, blaTEM-1, and blaPER-2), ESC (blaCTX-M-2 and blaPER-2), gentamicin [aac(3)-IIa and ant(2")-Ia], and amikacin [aac(6′)-Ib in class I integrons and in Tn1331] resistance in the Salmonella sp. strains isolated from nosocomial environments, where high antimicrobial pressure was exerted.
In a previous study (11), Tn1331 (36) was detected in several Salmonella serovars from 1989 to 1991. In this work, we found that this mechanism still plays an important role in the spreading of amikacin resistance since it was detected in the five serovars of isolates dating from 1991 to 1998. In Argentina, as in other countries, antibiotics can be bought over the counter, and it is well known that the natural bacterial gastrointestinal flora of our human population could act as a reservoir for the dissemination of resistance-conferring plasmids (R plasmids). This event could also contribute to the high level of antimicrobial resistance in Salmonella spp. isolated from nosocomial infections. In contrast to other opportunistic pathogens, Salmonella spp. are causative agents of zoonotic rather than nosocomial infections. In this regard, widespread use of antimicrobial agents in domestic animals of economic importance may have also contributed to increased levels of resistance in Salmonella spp.
Although the genus Salmonella has been described as having very stable plasmid profiles (38), several studies by others and the results from our research concluded that Salmonella serovar Typhimurium isolates as well as the other four serovars acquire and exchange R plasmids in the hospital microflora (1, 37, 38). On one hand similar, if not identical, conjugative R plasmids have been found in Salmonella serovar Typhimurium, E. coli, and S. flexneri strains that were coinfecting the same patients. On the other hand, evolution of R plasmids has been detected, as supported by the pattern found in the first period (1984 to 1988), and the accumulation of resistance determinants found during the second period (1989 to 1998). These findings not only indicate the possible source of antimicrobial resistance mechanisms but also suggest that intergenus exchange of plasmids, as well as accumulation of antimicrobial resistance determinants, is a common event that plays an important role in the evolution of multiresistant nontyphoid Salmonella species in Argentina.
Acknowledgments
We are very grateful to Ada Foguelman, Mabel Woloj, Sara Kaufman, and Mercedes Iglesias for providing several of the Salmonella spp. isolates.
This work was supported by a grant from Fundación A. Roemmers, Buenos Aires, Argentina, to D.C.
D. O. Sordelli and D. Centrón share the position of last author in this article.
REFERENCES
- 1.Archambaud, M., G. Gerbaud, E. Labau, N. Marty, and P. Courvalin. 1991. Possible in-vivo transfer of β-lactamase TEM-3 from Klebsiella pneumoniae to Salmonella kedougou. J. Antimicrob. Chemother. 27:427-436. [DOI] [PubMed] [Google Scholar]
- 2.Arduino, S. M., P. H. Roy, G. A. Jacoby, B. E. Orman, S. A. Piñeiro, and D. Centrón. 2002. blaCTX-M-2 is located in an unusual class 1 integron (In35) which includes Orf513. Antimicrob. Agents Chemother. 46:2303-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balis, E., A. C. Vatopoulos, M. Kanelopoulou, E. Mainas, G. Hatzoudis, V. Kontogianni, H. Malamou-Lada, S. Kitsou-Kiriakopoulou, and V. Kalapothaki. 1996. Indications of in vivo transfer of an epidemic R plasmid from Salmonella enteriditis to Escherichia coli of the normal human gut flora. J. Clin. Microbiol. 34:977-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauernfeind, A., Y. Stemplinger, R. Jungwirth, P. Mangold, S. Amann, E. Akalin, O. Ang, C. Bal, and J. M. Casellas. 1996. Characterization of β-lactamase gene blaPER-2, which encodes an extended-spectrum class A β-lactamase. Antimicrob. Agents Chemother. 40:616-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauernfeind, A., Y. Stemplinger, R. Jungwirth, S. Ernst, and J. M. Casellas. 1996. Sequences of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other β-lactamases. Antimicrob. Agents Chemother. 40:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford, P. A., Y. Yang, D. Sahm, I. Grope, D. Gardovska, and G. Storch. 1998. CTX-M-5, a novel cefotaxime-hydrolyzing β-lactamase from an outbreak of Salmonella typhimurium in Latvia. Antimicrob. Agents Chemother. 42:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush, K., G. A. Jacoby, and A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantón, R., A. Oliver, T. M. Coque, M. C. Varela, J. C. Pérez-Díaz, and F. Baquero. 2002. Epidemiology of extended-spectrum β-lactamase-producing Enterobacter isolates in a Spanish hospital during a 12-year period. J. Clin. Microbiol. 40:1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carattoli, A., L. Villa, C. Pezzella, E. Bordi, and P. Visca. 2001. Expanding drug resistance through integron acquisition by IncF1 plasmids of Salmonella enterica serovar Typhimurium. Emerg. Infect. Dis. 7:444-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casin, I., J. Breuil, A. Brisabois, F. Moury, F. Grimont, and E. Collatz. 1999. Multidrug-resistant human and animal Salmonella Typhimurium isolates in France belong predominantly to a DT104 clone with the chromosome- and integron-encoded β-lactamase PSE-1. J. Infect. Dis. 179:1173-1182. [DOI] [PubMed] [Google Scholar]
- 11.Centrón García, D., M. Woloj, S. Kaufman, D. O. Sordelli, and S. Piñeiro. 1995. Sequences related to Tn1331 associated with an unusual antimicrobial resistance pattern in different Salmonella serovars. Int. J. Antimicrob. Agents 5:199-202. [DOI] [PubMed] [Google Scholar]
- 12.Coque, T. M., O. Oliver, J. C. Pérez-Díaz, F. Baquero, and R. Cantón. 2002. Genes encoding TEM-4, SHV-2, and CTX-M-10 extended-spectrum β-lactamases are carried by multiple Klebsiella pneumoniae clones in a single hospital (Madrid, 1989 to 2000). Antimicrob. Agents Chemother. 46:500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordano, A. M., and R. Virgilio. 1996. Evolution of drug resistance in Salmonella panama isolates in Chile. Antimicrob. Agents Chemother. 40:336-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta, N., and V. M. Hughes. 1983. Plasmids of the same Inc groups in Enterobacteria before and after the medical use of antibiotics. Nature 306:616-617. [DOI] [PubMed] [Google Scholar]
- 15.Di Conza, J., J. A. Ayala, P. Power, M. Mollerach, and G. Gutkind. 2002. Novel class 1 integron (InS21) carrying blaCTX-M-2 in Salmonella enterica serovar Infantis. Antimicrob. Agents Chemother. 46:2257-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domenico, P., J. L. Marx, P. E. Scoch, and B. A. Cunha. 1992. Rapid plasmid DNA isolation from mucoid gram-negative bacteria. J. Clin. Microbiol. 30:2859-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunne, E. F., P. D. Fey, P. Kludt, R. Reporter, F. Mostashari, P. Shillam, J. Wicklund, C. Miller, B. Holland, K. Stamey, T. J. Barrett, J. K. Rasheed, F. C. Tenover, E. M. Ribot, and F. J. Angulo. 2000. Emergence of domestically acquired ceftriaxone-resistant Salmonella infections associated with AmpC β-lactamase. JAMA 27:3151-3156. [DOI] [PubMed] [Google Scholar]
- 18.Gazouli, M., E. Tzelepi, S. V. Sidorenko, and L. S. Tzouvelekis. 1998. Sequence of the gene encoding a plasmid-mediated cefotaxime-hydrolyzing class A β-lactamase (CTX-M-4): involvement of serine 237 in cephalosporin hydrolysis. Antimicrob. Agents Chemother. 42:1259-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glynn, M. K., C. Bopp, W. Dewitt, P. Dabney, M. Mokhtar, and F. J. Angulo. 1998. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N. Engl. J. Med. 338:1333-1338. [DOI] [PubMed] [Google Scholar]
- 20.González Leiza, M., J. C. Pérez-Díaz, J. Ayala, J. M. Casellas, J. Martínez-Beltrán, K. Bush, and F. Baquero. 1994. Gene sequence and biochemical characterization of FOX-1 from Klebsiella pneumoniae, a new AmpC-type plasmid-mediated β-lactamase with two molecular variants. Antimicrob. Agents Chemother. 38:2150-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerra, B., A. Soto, S. Cal, and C. Mendoza. 2000. Antimicrobial resistance and spread of class 1 integrons among Salmonella serotypes. Antimicrob. Agents Chemother. 44:2166-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, C., and J. Stanley. 1992. Salmonella plasmids of the pre-antibiotic era. J. Gen. Microbiol. 138:189-197. [DOI] [PubMed] [Google Scholar]
- 23.Lévesque, C., L. Piché, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llanes, C., V. Kirchgesner, and P. Plesiat. 1999. Propagation of TEM- and PSE-type β-lactamases among amoxicillin-resistant Salmonella spp. isolated in France. Antimicrob. Agents Chemother. 43:2430-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiorini, E., E. L. López, A. L. Morrow, F. Ramirez, A. Procopio, S. Furmanski, G. M. Woloj, G. Miller, and T. G. Cleary. 1993. Multiply resistant nontyphoidal Salmonella gastroenteritis in children. Pediatr. Infect. Dis. J. 12:139-145. [DOI] [PubMed] [Google Scholar]
- 27.Morosini, M. I., J. Blazquez, M. C. Negri, R. Cantón, E. Loza, and F. Baquero. 1996. Characterization of a nosocomial outbreak involving an epidemic plasmid encoding for TEM-27 in Salmonella enterica subspecies enterica serotype Othmarschen. J. Infect. Dis. 174:1015-1020. [DOI] [PubMed] [Google Scholar]
- 28.Nastasi, A., and C. Mammina. 2001. Presence of class I integrons in multidrug-resistant, low-prevalence Salmonella serotypes in Italy. Emerg. Infect. Dis. 7:455-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 30.Olsen, S. J., R. Bishop, F. W. Brenner, T. H. Roels, N. Bean, R. V. Tauxe, and L. Slutsker. 2001. The changing epidemiology of Salmonella: trends in serotypes isolated from humans in the United States, 1987-1997. J. Infect. Dis. 183:753-761. [DOI] [PubMed] [Google Scholar]
- 31.Parsons, Y., R. M. Hall, and H. W. Stokes. 1991. A new trimethoprim resistance gene, dhfrX, in the In7 integron of plasmid pDGO100. Antimicrob. Agents Chemother. 35:2436-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popoff, M. Y. 2001. Antigenic formula of the Salmonella serovars, 8th ed. Institut Pasteur, Paris, France.
- 33.Rossi, M. A., M. Tokumoto, E. Couto, A. Di Bella, M. Alstchuler, N. Gómez, F. Dujovney, L. Galanternik, M. Woloj, N. Hardie, J. Stellin, M. Schlipak, and T. O'Brien. 1995. Survey of the levels of antimicrobial resistance in Argentina: WHONET program—1991 to 1994. Int. J. Antimicrob. Agents 6:103-110. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Stokes, H. W., C. Tomaras, Y. Parsons, and R. M. Hall. 1993. The partial 3′ conserved segment duplications in the integron In6 from pSa and In7 from pDGO100 have a common origin. Plasmid 30:39-50. [DOI] [PubMed] [Google Scholar]
- 36.Tolmasky, M. E. 1990. Sequencing and expression of aadA, bla, and tnpR from the multiresistance transposon Tn1331. Plasmid 24:218-226. [DOI] [PubMed] [Google Scholar]
- 37.Tosini, F., P. Visca, I. Luzzi, A. M. Dionisi, C. Pezzella, A. Petrucca, and A. Carattoli. 1998. Class 1 integron-borne multiple-antibiotic resistance carried by IncF1 and IncL/M plasmids in Salmonella enterica serotype Typhimurium. Antimicrob. Agents Chemother. 42:3053-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vahaboglu, H., S. Dodanli, C. Eroglu, R. Ozturk, G. Soyletir, I. Yildrim, and V. Avkan. 1996. Characterization of multiple-antibiotic-resistant Salmonella typhimurium strains: molecular epidemiology of PER-1-producing isolates and evidence for nosocomial plasmid exchange by a clone. J. Clin. Microbiol. 34:2942-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vahaboglu, H., M. Fuzi, S. Cetin, S. Gundes, E. Ujhelyi, F. Coskunkan, and O. Tansel. 2001. Characterization of extended-spectrum β-lactamase (TEM-52)-producing strains of Salmonella enterica serovar Typhimurium with diverse resistance phenotypes. J. Clin. Microbiol. 39:791-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verdet, C., G. Arlet, G. Barnaud, P. H. Lagrange, and A. Philippon. 2000. A novel integron in Salmonella enterica serovar Enteritidis, carrying the blaDHA-1 gene and its regulator gene ampR, originated from Morganella morganii. Antimicrob. Agents Chemother. 44:222-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wegener, H. C., and D. L. Baggesen. 1996. Investigation of an outbreak of human salmonellosis caused by Salmonella enterica serovar Infantis by use of pulsed-field gel electrophoresis. Int. J. Food Microbiol. 32:125-131. [DOI] [PubMed] [Google Scholar]