Abstract
To characterize the potential of ciprofloxacin penetration into human soft tissues following intravenous (i.v.) and oral (p.o.) administration, we measured the free ciprofloxacin concentrations in interstitial space fluid of skeletal muscle and subcutaneous adipose tissue by microdialysis. In addition, ciprofloxacin concentrations were measured in cantharis-induced skin blisters, saliva, and capillary plasma and were compared to the total concentrations in venous plasma. Furthermore, a pharmacodynamic in vitro model was used to simulate in vivo pharmacokinetics in bacterial culture. Eight healthy volunteers received ciprofloxacin in an open randomized crossover fashion either as a single i.v. infusion of 400 mg over 60 min or as a single p.o. dose of 500 mg. For both tissues the mean areas under the concentration-time curves (AUCs) for interstitial space fluid (AUCinterstitial fluids) were significantly lower than the corresponding AUCplasmas, with AUCinterstitial fluid/AUCplasma ratios ranging from 0.38 to 0.68. For skeletal muscle, the AUCinterstitial fluid was significantly higher after administration of 400 mg i.v. than after administration of 500 mg p.o., with a ratio of the AUC after p.o. administration/AUC after i.v. administration of 0.64. The ratio of the concentration in skeletal muscle/concentration in plasma increased over the entire observation period, implying that ciprofloxacin concentrations were not at steady state. The ratio of the concentration in skin blister fluid/concentration in plasma reached values above 4, indicating a preferential penetration of ciprofloxacin into inflamed lesions. The concentrations in saliva and capillary blood were similar to the corresponding total levels in plasma. In vitro both in vivo ciprofloxacin concentration-time profiles were equally effective against select bacterial strains. In conclusion, single-dose administration of two bioequivalent dosage forms of ciprofloxacin might lead to differences in target site pharmacokinetics. These differences, however, are not related to a difference in target site pharmacodynamics.
Ciprofloxacin is one of the few fluoroquinolone antibiotics which is available as a parenteral and an oral (p.o.) formulation. Due to its broad spectrum of antibacterial activity and its favorable pharmacokinetic profile and bioavailability, sequential intravenous (i.v.) and p.o. therapy is increasingly applied in the treatment of infections which have traditionally required parenteral antibiotic therapy (5). However, in order not to risk a decrease in bactericidal potential when a switch from i.v. to p.o. formulations is performed, it must be ensured that both formulations are equally effective. In particular, equivalent antibiotic concentrations should be attained in the interstitial space fluid, since for most infections the interstitial space rather than the bloodstream represents the biophase, i.e., the location of the interaction between the antibiotic and the pathogen (8). Thus, the availability of ciprofloxacin at the interstitial target site is considered an important determinant for the effectiveness of antimicrobial therapy and the clinical outcome of an infection.
The relevance of achieving therapeutic antibiotic concentrations in the interstitial space is furthermore highlighted by the fact that target site concentrations might be substantially lower than the total concentrations in tissue and may even be severalfold below the corresponding concentrations in plasma (12), since the distribution of antimicrobial agents to a peripheral compartment is influenced by factors such as the degree of plasma protein binding, local blood flow, vascular permeability, and the local surface area-to-volume ratio (8). Thus, a standard i.v. or p.o. dose of ciprofloxacin might lead to subinhibitory concentrations at the target site, which were claimed to be responsible for promoting the emergence of resistant bacterial strains (6). Furthermore, subinhibitory concentrations at the target site might also provide an explanation for those cases in which ciprofloxacin failed to eradicate the relevant pathogen, despite documented in vitro susceptibility (9).
Therefore, data on the ability of ciprofloxacin to penetrate peripheral target sites following the administration of i.v. and p.o. formulations would allow selection of those dosing schedules which achieve equal concentrations at the target site. On the basis of equivalent concentrations at the target site, a switch from i.v. to p.o. antibiotic therapy might be initiated, promoting an earlier discharge of hospitalized patients combined with a reduction in expenditures on antimicrobial agents (15). A switch from i.v. to p.o. therapy with ciprofloxacin has been reported to result in cost savings in the treatment of several serious infections, such as respiratory or urinary tract infections, infections of the bone and joints, as well as soft tissue infections (1).
The present study was conducted to compare the ciprofloxacin concentrations in the interstitial space fluid of two peripheral target sites, skeletal muscle and subcutaneous adipose tissue, following the administration of 400-mg i.v. and 500-mg p.o. formulations of ciprofloxacin. Additionally, the time-versus-concentration profiles of ciprofloxacin were monitored in cantharis-induced skin blisters, capillary blood, and saliva and were compared to the concentrations in venous plasma.
MATERIALS AND METHODS
The study was approved by the local ethics committee. All volunteers were given a detailed description of the study, and their written consent was obtained. The study was performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guideline of the European Commission.
Healthy volunteers.
The study population included eight healthy, drug-free male volunteers of normal weight (mean weight, 82 kg; weight range, 73 to 96 kg) between 22 and 37 years old. Each volunteer was subjected to a screening examination including medical history, physical examination, 12-lead electrocardiogram, blood pressure, complete blood count with differential blood analysis, urinalysis, urine drug screen, clinical blood chemistry analysis, blood coagulation tests, an HBs antigen test, and a human immunodeficiency virus antibody test. Subjects were excluded if they had taken any prescribed medication or over-the-counter drugs within a period of 2 weeks prior to the study. For each study day, the volunteers were kept under fasting conditions from 10 h prior to the start of the experiments until 2 h after drug administration.
Overall study design and plan of trial.
The study was conducted as a single-center, single-dose, non-placebo-controlled, randomized, non-blinded, crossover trial with eight healthy young male volunteers. Each volunteer was studied twice and was randomly assigned to receive either ciprofloxacin at 500 mg p.o. as a tablet or ciprofloxacin at 400 mg i.v. as an infusion over 60 min on each occasion after an overnight fast of 10 h. The pharmacokinetics of ciprofloxacin in microdialysates of skeletal muscle and subcutaneous adipose tissue, saliva, capillary and venous plasma, urine, and cantharis-induced skin blisters were measured as described in detail below. Volunteers were hospitalized from the morning before the administration of ciprofloxacin until 25 h postdosing. There was a washout phase of at least 1 week between the two treatments.
Sampling by microdialysis.
To measure the unbound fraction of ciprofloxacin in the interstitial space fluid, custom-made microdialysis probes (CMA 10; CMA, Stockholm, Sweden) with a molecular cutoff of 20 kDa were used. The principles of microdialysis have previously been described in detail (3, 11, 12) Briefly, microdialysis is based on sampling of the non-protein-bound fraction and, therefore, the pharmacologically active fraction of analytes from the interstitial space with a semipermeable membrane at the tip of a microdialysis probe. The probe is constantly perfused with a physiological solution (perfusate) at a flow rate of 1.5 μl/min. Once the probe is implanted into the tissue, substances present in the extracellular fluid at a particular concentration (Ctissue) are filtered out of the interstitial space fluid by perfusion into the probe, resulting in a concentration (Cdialysate) in the perfusate. Samples are collected and analyzed. For most analytes, equilibrium of the concentration between extracellular tissue fluid and the perfusion medium is incomplete; therefore, Ctissue > Cdialysate. The factor by which the concentrations are interrelated is termed recovery.
Assessment of microdialysis probe recovery.
To obtain absolute concentrations in the interstitial space fluid from the concentrations in unbound dialysate, microdialysis probes were calibrated for in vivo recovery rates by the retrodialysis method (17). The principle of this method relies on the assumption that the diffusion process is quantitatively equal in both directions through the semipermeable membrane. Therefore, ciprofloxacin was added to the perfusate at a concentration of 0.1 mg/liter, and the disappearance rate (delivery) through the membrane was taken as the in vivo recovery. The in vivo percent recovery was calculated as 100 − (100 · Cdialysate of the analyte · Cperfusate of the analyte−1).
Microdialysis probes were inserted after cleaning and thorough disinfection of the skin. One dialysis probe was inserted into the medial vastus muscle and one was inserted into the subcutaneous layer of the thigh by a previously described procedure (12). The microdialysis system was perfused with Ringer's solution at a flow rate of 1.5 μl/min with a microinfusion pump (Precidor; Infors-AG, Basel, Switzerland); during the in vivo calibration periods, however, the system was perfused with a stock solution containing Ringer's solution and ciprofloxacin. After a 30-min baseline perfusion period, in vivo calibration was performed as described previously for a period of 30 min, during which two samples were collected at 15 min, followed by a 30-min washout period.
Skin blister fluid sampling.
Cantharis-induced skin blisters are caused by a toxic reaction that leads to the formation of a subepidermally located blister. Cantharis-impregnated plasters were used to induce skin blisters as described previously (13). An ointment containing 0.25% cantharidin was prepared by mixing pure cantharidin with ointment base. On the evening before puncture of the blisters (−12 h), eight 0.25% cantharis-impregnated plasters (1 by 1 cm) were applied to the abdominal skin of each subject. The patches of polyethylene sheeting were held in place with adhesive tape over 12 h, leading to the formation of blisters within 12 h. On the study day, approximately 1 ml of the blister fluid was aspirated into a syringe by puncturing the blister with a fine needle at defined time points. The blister fluid was placed into Eppendorf cups and was immediately stored frozen in an upright position at −80°C.
Sampling of capillary and venous plasma.
For sampling of capillary plasma, two finger pads were pricked with a lance. Subsequently, blood was sampled with a pipette and transferred into Eppendorf cups for centrifugation. Within 10 min the capillary blood was centrifuged at 1,600 × g for 5 min at 5°C. The plasma was then pipetted into polypropylene tubes and immediately frozen in an upright position. For sampling of venous plasma, venous blood from a forearm vein was centrifuged within 10 min at 1,600 × g for 5 min at 5°C and immediately frozen in polypropylene tubes at −80°C.
Saliva sampling.
Commercially available sampling devices (Salivetten, Harstedt, Germany) were used for saliva sampling. Volunteers were requested to chew on special cotton wool rolls for 30 to 45 s at the appropriate sampling times. The cotton rolls were then transferred into plastic tubes, and screw caps were immediately placed on the tubes to avoid evaporation. Subsequently, the tubes were centrifuged at 1,000 × g for 5 min at 5°C to obtain at least 0.7 ml of saliva. The entire sampling device was immediately stored frozen at −80°C.
In vitro simulation experiments.
In order to generate a pharmacodynamic model which allows description of the antibacterial activity of ciprofloxacin in the interstitial space fluid, we simulated the time-versus-concentration profile of ciprofloxacin in the interstitial space fluid in vitro, based on the pharmacokinetic data obtained from the in vivo experiments. Therefore, 50-ml Falcon tubes with 2 ml of Mueller-Hinton broth (MHB) were kept in a water bath at 37°C and were then inoculated with select bacteria commonly found in soft tissue infections at an approximate concentration of 106 CFU/ml. Subsequently, the time-versus-ciprofloxacin concentration profile obtained in vivo from data on the concentration in interstitial space fluid was simulated in vitro by changing the ciprofloxacin concentrations in broth by adding the appropriate amount of MHB at 20-min intervals, depending on the individual pharmacokinetic data, by the following equation: V2 = (C1/C2) × V1, where C1 and V1 represent the current ciprofloxacin concentration and the current MHB volume, respectively; C2 is the desired ciprofloxacin concentration; and V2 is the calculated volume of MHB to be added to simulate the ciprofloxacin clearance in each simulation.
Samples for determination of bacterial counts were drawn at fixed time points (0, 45, 105, 165, 225, 285, 360, 435, 495, 555, 612, and 720 min). Ciprofloxacin concentrations used for simulation were not directly controlled, e.g., by high-pressure liquid chromatography (HPLC). However, to ensure that the pharmacokinetic profile in vitro resembled the concentration profile observed in vivo, a strict experimental protocol was followed. The protocol was designed as follows to eliminate potential sources of error: (i) each simulation was performed in triplicate; (ii) individual simulations including ciprofloxacin preparation were performed by two different investigators, (iii) fresh ciprofloxacin stock solutions were prepared for each simulation, and (iv) to exclude contamination, fresh bacterial cultures were used for each simulation.
In vitro susceptibility tests.
The MICs of ciprofloxacin were determined by a twofold serial microdilution method in MHB. Strains of Enterobacter, Klebsiella pneumoniae, and Staphylococcus aureus were precultured overnight in brain heart infusion broth and were then introduced into MHB containing ciprofloxacin at an inoculum of approximately 5 × 105 CFU/ml. The lowest concentration of ciprofloxacin which inhibited bacterial growth after incubation for 24 h at 37°C was considered the MIC. The minimal bactericidal concentration (MBC) was determined by subculturing 20 μl of the broth onto antibiotic-free Columbia agar plates. The lowest antibiotic concentration which did not show any visible growth after 24 h at 37°C was considered the MBC. The MBCs for the study strains were 1 dilution step higher than the respective MICs.
Organism.
The study strains were Enterobacter (MIC, 0.03 mg/liter), K. pneumoniae (MIC, 0.03 mg/liter), and S. aureus (MIC, 2 mg/liter). The strains were patient isolates and were identified by standard laboratory methods. All were stored frozen in liquid nitrogen at −196°C until they were used.
Pharmacodynamic analysis.
Samples for determination of bacterial counts were withdrawn at defined time points for up to 720 min. After the culture tube was vortexed, a 40-μl sample was pulled and serially diluted with 0.9% sodium chloride. Twenty microliters from each dilution step was then plated onto Columbia agar plates. The plates were then incubated at 37°C for 18 to 26 h. Subsequently, the colonies were counted and the number of CFU was backextrapolated to the original volume.
Analyses. (i) Bioanalysis.
Quantitative determination of ciprofloxacin in all body fluids was carried out by validated HPLC methods with fluorescence detection. The method showed accuracy and precision throughout the working range (interday precision, <9%; interday accuracy, 96 to 110%). The limit of quantification was 0.01 mg/liter (2).
(ii) Data analysis.
The absolute concentrations in interstitial space fluid were calculated from the concentrations in unbound dialysate by the following equation: concentration in interstitial space fluid = 100 · concentration in sample · in vivo recovery value−1.
Primary noncompartmental pharmacokinetic parameters (area under the concentration-time curve [AUC], maximum concentration of drug [Cmax]), half-life [t1/2], and time to Cmax [Tmax]) were calculated by using KINCALC software (BAYER AG, Wuppertal, Germany). All data are presented as geometric means ± standard deviations.
As the pharmacokinetic parameters were nonnormally distributed, Mann-Whitney U tests were used for comparisons between groups. For comparisons within groups, Wilcoxon matched-pairs tests were used. A P value <0.05 was considered the level of significance.
RESULTS
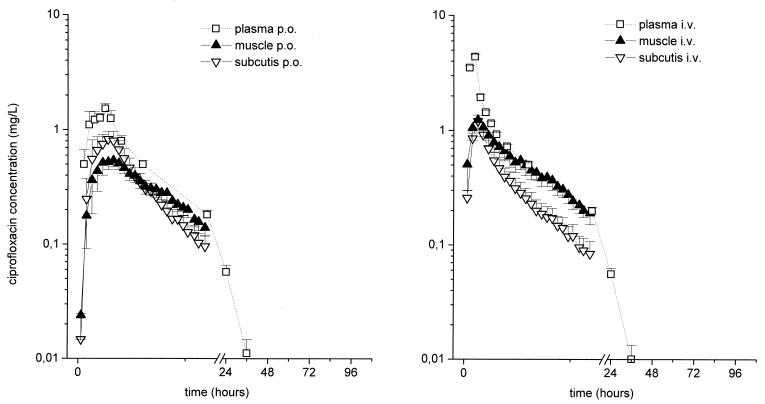
The results of experiments in which probes were inserted simultaneously into the medial vastus muscle and the subcutaneous adipose tissue of healthy volunteers following the administration of 400-mg i.v. and 500-mg p.o. formulations of ciprofloxacin indicate that the concentrations in the interstitial space fluid at the target sites and AUCs were significantly lower than the corresponding concentrations in plasma for both dosages (P = 0.01) (Table 1; Fig. 1), with the ratios of the concentration in the interstitial space fluid/concentration in plasma being between 0.24 and 0.99 (see Fig. 3). In particular, the ratios of the AUC for the peripheral compartment (AUCperipheral compartment)/AUCplasma were 0.57 ± 0.04 and 0.57 ± 0.09 for muscle and subcutis, respectively, after p.o. administration and 0.68 ± 0.08 and 0.38 ± 0.05, respectively, after i.v. administration. Statistical analysis did not reveal a period effect in the present crossover study.
TABLE 1.
Pharmacokinetic parameters for venous plasma (0 to 96 h), skeletal muscle and subcutaneous adipose tissue interstitial fluid (0 to 12 h), saliva (0 to 96 h), capillary plasma (0 to 72 h), and cantharis-induced skin blister fluid (0 to 24 h) following administration of ciprofloxacin to healthy volunteersa
| Route of administration and compartment | AUC (mg · h · liter−1) | Cmax (mg · liter−1) | Tmax (h) | t1/2 (h) | Clearance (liter · h−1) |
|---|---|---|---|---|---|
| i.v. | |||||
| Plasma | 12.10 (1.15)b | 4.34 (1.14)b | 1.00 (1.00) | 5.99 (1.11)b | 33.10 (1.15)b |
| Muscle | 7.43 (1.40) | 1.24 (1.72) | 1.10 (1.27) | 4.82 (1.32) | 53.80 (1.40) |
| Subcutis | 4.13 (1.63)b | 1.18 (1.40)b | 1.17 (1.20) | 3.90 (1.18)b,c | 96.90 (1.63)b,c |
| Saliva | 5.39 (1.32) | 2.52 (1.25) | 1.16 (1.24) | 3.55 (1.50) | 74.10 (1.32) |
| Capillary | 11.20 (1.17) | 3.86 (1.15) | 1.00 (1.00) | 5.77 (1.12) | 35.80 (1.17) |
| Blister | 17.80 (1.17) | 1.40 (1.44) | 1.36 (1.21) | 8.92 (1.52)c | 22.50 (1.17)c |
| p.o. | |||||
| Plasma | 8.89 (1.22)b | 1.87 (1.27)b | 1.86 (1.54)b | 6.56 (1.10)b | 56.20 (1.22)b |
| Muscle | 4.49 (1.41) | 0.62 (1.63) | 2.74 (1.52) | 4.31 (1.41) | 111.00 (1.41) |
| Subcutis | 3.85 (2.26)b | 0.84 (2.36)b | 2.45 (1.41)b,c | 3.56 (1.38)b,c | 130.00 (2.26)b,c |
| Saliva | 2.95 (1.32) | 0.95 (1.23) | 2.17 (1.50) | 2.85 (1.71) | 169.00 (1.32) |
| Capillary | 7.63 (1.32) | 1.30 (1.61) | 1.29 (1.23) | 6.05 (1.01) | 65.50 (1.32) |
| Blister | 12.60 (1.42) | 0.56 (1.51) | 4.91 (2.67)c | 11.80 (1.48)c | 39.60 (1.42)c |
The results are presented as geometric means (standard deviations). Ciprofloxacin was administered to eight healthy volunteers as a 400-mg i.v. dose over 60 min or a 500-mg tablet p.o.
P < 0.05 versus result for plasma.
P < 0.05 versus result for blister fluid.
FIG. 1.
Time versus total concentration in plasma and unbound concentration in interstitial space fluid profiles for muscle and subcutaneous adipose tissue following administration of a single p.o. dose of 500 mg of ciprofloxacin or a single i.v. dose of 400 mg to healthy volunteers (n = 8). The results are presented as means ± standard errors. The time of drug administration was from 0 to 1 h.
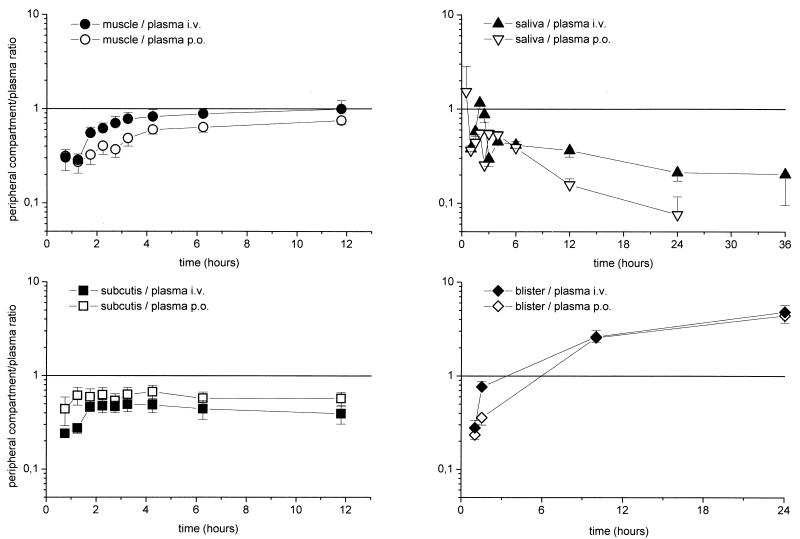
FIG. 3.
Time course of ratios of the ciprofloxacin concentration in the interstitial space fluid of muscle or subcutaneous tissue, saliva, and cantharis-induced skin blister fluid to the concentration in plasma for the experiments shown in Fig. 1. The results are presented as means ± standard errors. The time of drug administration was from 0 to 1 h.
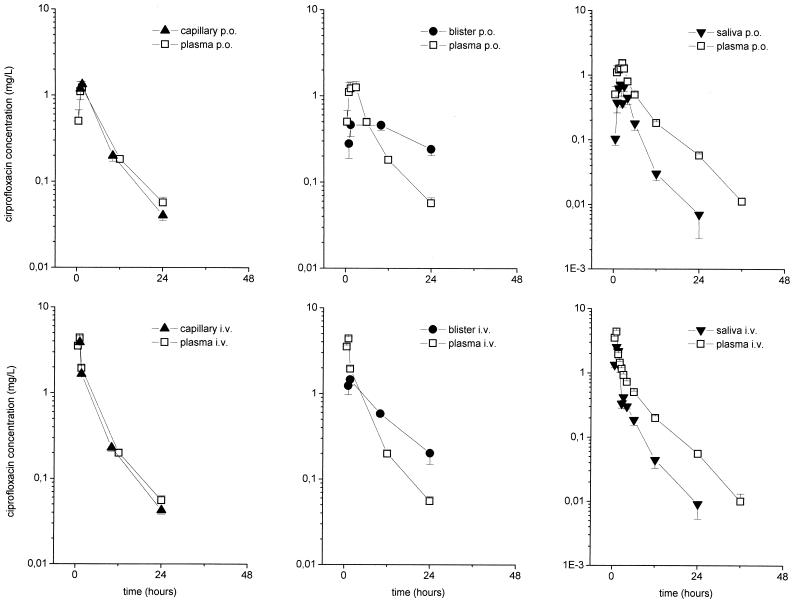
The time-versus-concentration profiles of ciprofloxacin in capillary blood, skin blister fluid, and saliva are shown in Fig. 2. The ciprofloxacin concentrations in capillary plasma and saliva closely reflected the corresponding concentrations in venous plasma. There were significant differences between the AUCs after i.v. and p.o. administration (P = 0.006 and 0.003, respectively). The results of the experiments with skin blister fluid indicate the accumulation of ciprofloxacin in blister fluid due to a third-compartment effect (Fig. 3). The time courses of the ratios of the concentration in the peripheral compartment/concentration in plasma are depicted in Fig. 3. In particular, after p.o. administration, the AUCsaliva/AUCplasma ratio was 0.33 ± 0.01, the AUCcapillary blood/AUCplasma ratio was 0.88 ± 0.07, and the AUCskin blister/AUCplasma ratio was 1.44. ± 0.16. After i.v. administration, the AUCsaliva/AUCplasma ratio was 0.46 ± 0.03, the AUCcapillary blood/AUCplasma ratio was 0.93 ± 0.03, and the AUCskin blister/AUCplasma ratio was 1.46. ± 0.09.
FIG. 2.
Profiles of time versus concentrations in plasma, capillary blood, cantharis-induced skin blister fluid, and saliva following administration of a single p.o. dose of 500 mg of ciprofloxacin (upper panels) or a single i.v. dose of 400 mg over 60 min (lower panels) to healthy volunteers (n = 8). The results are presented as means ± standard errors. The time of drug administration was from 0 to 1 h.
The AUCperoral/AUCintravenous ratio was 0.74 ± 0.03 for plasma (P = 0.014 between groups); 0.64 ± 0.08 (P = 0.016) and 1.30 ± 0.28 (P = 0.67) for muscle and subcutis, respectively; and 0.56 ± 0.05 (P = 0.003), 0.71 ± 0.07 (P = 0.006), and 0.75 ± 0.10 (P = 0.16) for saliva, capillary blood, and skin blister fluid, respectively. Pharmacokinetic data for interstitial space fluid in skeletal muscle and subcutaneous adipose layer, saliva, capillary blood, cantharis-induced skin blister fluid, and plasma are given in Table 1.
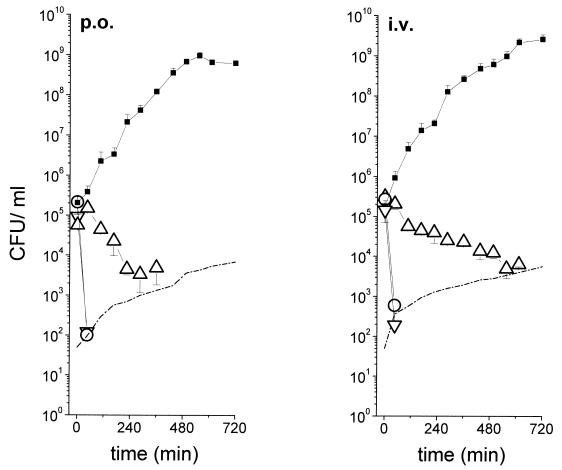
Mean time-kill curves for the in vivo pharmacokinetic-in vitro pharmacodynamic simulation model are shown in Fig. 4. The curves indicate comparable pharmacodynamics after 12 h of simulation for ciprofloxacin given at 400 mg i.v. and 500 mg p.o.
FIG. 4.
Mean time-kill curves (n = 6) for the in vivo pharmacokinetic-in vitro pharmacodynamic simulation model. The in vivo time course of the ciprofloxacin concentration was obtained by measuring the antibiotic concentrations in the interstitial space fluid of subcutaneous adipose tissue for the experiments shown in Fig. 1 after administration of 500 mg of ciprofloxacin p.o. and 400 mg of ciprofloxacin i.v. The in vitro simulation was done with select strains of Enterobacter (▿), K. pneumoniae (○), and S. aureus (▵). ▪, control growth curves; dotted lines, detection limit.
DISCUSSION
After several years of clinical experience with ciprofloxacin, ciprofloxacin has retained its excellent activity against most gram-negative bacteria and has fulfilled its potential as an important antibacterial drug in the treatment of a wide range of infections (20). However, the emergence of resistance in pathogens previously exquisitely sensitive to ciprofloxacin has been noted worldwide (18). Whereas spontaneous mutations which confer resistance to ciprofloxacin are usually infrequent (10), a large inoculum of pathogens at the target site and poor penetration of the drug into the infected tissue at the target site have been noted to promote the emergence of resistant bacterial strains (10). Therefore, data on the penetration potential of ciprofloxacin to peripheral target sites are crucial, since the drug concentrations at the focus of infection are among other factors decisive for efficacy, i.e., eradication of the causative pathogens.
To investigate the penetration potentials of two different dosage forms of ciprofloxacin to relevant target sites of soft tissue infections, the concentration of the non-protein-bound fraction of ciprofloxacin was measured in skeletal muscle and subcutaneous adipose tissue by the microdialysis technique after the administration of a single i.v. or p.o. dose of 400 or 500 mg of ciprofloxacin, respectively. In both target tissues, the time-versus-concentration profiles showed that the free concentrations in interstitial space fluid were lower than the corresponding total concentrations in plasma; and the profiles paralleled those in plasma after i.v. and p.o. administration (Fig. 1), with the ratios of the concentration in interstitial space fluid/concentration in plasma being between 0.24 and 0.99 (Fig. 3). The profiles of the concentration in interstitial space fluid were not absolutely congruent, a finding that has been observed previously (3) and that might be explained by differences in local blood flow between the two tissues.
Based on studies reporting on the equivalence between the 400-mg i.v. dose and the 500-mg p.o. dose (7), the Food and Drug Administration has approved the 400-mg i.v. dose for those indications for which the 500-mg p.o. dose was recommended. The absolute bioavailability measured in the present study (∼58%; published range, 56 to 77%) thus confirms the findings in the literature that the 400-mg i.v. dose equals the 500-mg p.o. dose and may also allow one to conclude that the two formulations are equivalent on the basis of international guidelines. The Food and Drug Administration approval was preceded by the determination that the AUC for plasma is the critical parameter for the establishment of bioequivalence. However, from a bacteriologic standpoint, AUCs for the target sites should be compared to draw conclusions related to the bactericidal potentials of both dosage forms. Furthermore, although ciprofloxacin is known to exhibit favorable penetration into tissue, the extent to which AUCs for the target site differ from the AUCs for plasma after i.v. and p.o. administration has not yet been evaluated. After the administration of single i.v. and p.o. doses of 400 and 500 mg ciprofloxacin, respectively, the mean AUCs for the interstitial space fluid in muscle and adipose tissue were significantly lower than the corresponding AUCs for plasma. The AUCs for the interstitial space fluid in skeletal muscle after the administration of 400 mg i.v. were significantly higher than the AUCs after the administration of 500 mg p.o., with a ratio of the AUC after p.o. administration/AUC after i.v. administration of 0.6, whereas no significant difference could be detected between the two formulations by measurement of the AUCs for the interstitial space fluid in adipose tissue. Although for adipose tissue the ratios of the concentration in the peripheral compartment/concentration in plasma indicate equilibration of the concentrations in the interstitial space fluid after p.o. and i.v. administration (Fig. 3), the ratio of the concentration in skeletal muscle/concentration in plasma increases during the entire observation period and reaches values above those of the non-protein-bound ciprofloxacin fraction. Those findings promote the notion that after the administration of a single dose of ciprofloxacin, the concentrations in skeletal muscle are not at steady state.
To investigate whether the reported differences in the pharmacokinetics of both dosage forms in peripheral tissue had an impact on the bactericidal activity at the target site, we used a previously described in vivo pharmacokinetic-in vitro pharmacodynamic simulation model (3). Briefly, bacterial strains commonly isolated from soft tissue infections, such as S. aureus, Enterobacter, and K. pneumoniae, were exposed in vitro to ciprofloxacin concentrations previously determined by in vivo microdialysis and subsequently bactericidal kinetics were assessed (Fig. 4). Subcutaneous in vivo pharmacokinetics were simulated to exemplify the situation in a soft tissue. After a 12-h simulation, the numbers of CFU were below the detection limits for all three strains, a finding which implies eradication of the pathogens and therefore underlines the notion that both formulations are equivalent not only from a pharmacokinetic point of view but also from a pharmacodynamic point of view under the given experimental conditions, even though the concentrations in tissue suggest that there is no steady state after single-dose application. It must be noticed that the growth kinetics for the S. aureus strain were unexpected, because the MIC was higher than Cmaxs in the interstitial space fluid after both i.v. and p.o. dosing, indicating a bactericidal effect rather than growth inhibition. Although experiments were performed according to a strict experimental protocol to minimize potential sources of error, there is no ready explanation for this discrepancy, and the possibility that the drug concentrations in the simulation were actually higher than expected cannot be completely ruled out.
For capillary plasma, ratios of the concentration in capillary plasma/concentration in plasma of 0.9 and 0.8 after i.v. and p.o. administration, respectively, were observed (Fig. 3). AUCs and Cmaxs were significantly different between the i.v. and p.o. formulations, with an AUC ratio of 0.7 for the two formulations. The ciprofloxacin concentrations in saliva reflected the corresponding concentrations in venous plasma, with ratios of 0.5 and 0.3 after i.v. and p.o. administration, respectively, which are in accordance with those presented in another report (21). However, the AUCs after i.v. and p.o. administration were significantly different. In conclusion, these findings support the findings presented in previous reports that concentrations in saliva cannot be reliably used as a surrogate measure of the levels in plasma, a finding with clinical implications for the monitoring of therapeutic drug concentrations in patients with diseases such as cystic fibrosis (16).
For cantharis-induced skin blister fluid, the ratio of the concentration in skin blister fluid/concentration in plasma increased after an initial lag time and resulted in a ratio of approximately 4 at 24 h after both i.v. and p.o. administration, supporting the concept that fluoroquinolones may accumulate in an inflammatory environment (4, 19). Cantharis-induced skin blisters contain an inflammatory exudate. Thus, a part of the observed accumulation of ciprofloxacin may be due to the binding to blister fluid proteins (13). Furthermore, blister fluid contains macrophages, which have been shown to actively transport ciprofloxacin intracellularly to the site of an infection as part of the inflammatory response. Once they have reach their target region, either the macrophages might actively release the antibiotic or the antibiotic might be passively set free when the cells are destroyed. Several studies have described the accumulation of ciprofloxacin in inflammatory cells and its release at a site of infection (4, 19). A recently conducted clinical study with diabetic patients with foot infections, however, could not detect a substantial augmentation of ciprofloxacin concentrations in the interstitial space fluid of inflamed tissue (14). For ciprofloxacin, extrapolation of the data for the concentrations in skin blister fluid to the concentrations in infected tissues should be done with extreme caution. One problem is that skin blisters are formed before the administration of the antibiotic. The blister thus serves as a large third compartment with a surface-to-volume ratio which is hardly representative of that for tissue. Besides, other variables must be taken into account, such as the barrier between the blister and the skin, which may change over time; the presence of proteins in the blister fluid; or cell lysis with subsequent release of antibiotic during sample preparation.
The results of the present study indicate that administration of a single dose of two bioequivalent dosage forms of ciprofloxacin might lead to differences in pharmacokinetics at the target site. These differences, however, are not related to differences in pharmacodynamics at the target site.
REFERENCES
- 1.Amodio-Groton, M., A. Madu, C. N. Madu, L. L. Briceland, M. Seligman, P. McMaster, and M. H. Miller. 1996. Sequential parenteral and oral ciprofloxacin regimen versus parenteral therapy for bacteremia: a pharmacoeconomic analysis. Ann. Pharmacother. 30:596-602. [DOI] [PubMed] [Google Scholar]
- 2.Bannefeld, K. H., H. Stass, and G. Blaschke. 1997. Capillary electrophoresis with laser-induced fluorescence detection, an adequate alternative to high performance liquid chromatography, for the determination of ciprofloxacin and its metabolite desethyleneciprofloxacin in human plasma. J. Chromatogr. B 692:453-459. [DOI] [PubMed] [Google Scholar]
- 3.Brunner, M., U. Hollenstein, S. Delacher, D. Jäger, R. Schmid, E. Lackner, A. Georgopoulos, H. G. Eichler, and M. Müller. 1999. Distribution and antimicrobial activity of ciprofloxacin in human soft tissues. Antimicrob. Agents Chemother. 43:1307-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlier, M. B., B. Scorneaux, A. Zenebergh, J. F. Desnottes, and P. M. Tulkens. 1990. Cellular uptake, localization and activity of fluoroquinolones in uninfected and infected macrophages. J. Antimicrob. Chemother. 26(Suppl. B):27-39. [DOI] [PubMed] [Google Scholar]
- 5.Davis, R., A. Markham, and J. A. Balfour. 1996. Ciprofloxacin. An updated review of its pharmacology, therapeutic efficacy and tolerability. Drugs 51:1019-1074. [DOI] [PubMed] [Google Scholar]
- 6.Dudley, M. N., H. D. Mandler, D. Gilberd, J. Ericson, K. H. Mayer, and S. H. Zinner. 1987. Pharmacokinetics and pharmacodynamics of intravenous ciprofloxacin: studies in vivo and in an in vitro dynamic model. Am. J. Med. 82(Suppl. 4A):363-368. [PubMed] [Google Scholar]
- 7.Echols, R. M. 1993. The selection of appropriate dosages for intravenous ciprofloxacin. J. Antimicrob. Chemother. 31:783-787. [DOI] [PubMed] [Google Scholar]
- 8.Eichler, H. G., and M. Müller. 1998. Drug distribution. The forgotten relative in clinical pharmacokinetics. Clin. Pharmacokinet. 34:95-99. [DOI] [PubMed] [Google Scholar]
- 9.Fass, R. J. 1986. Treatment of skin and soft tissue infections with oral ciprofloxacin. J. Antimicrob. Chemother. 18(Suppl. D):153-157. [DOI] [PubMed] [Google Scholar]
- 10.Jones, R. N., E. N. Kehrberg, and M. E. Erwin. 1994. Prevalence of important pathogens and antimicrobial activity of parenteral drugs at numerous medical centers in the United States. I. Study on the threat of emerging resistances: real or perceived? Diagn. Microbiol. Infect. Dis. 19:203-215. [DOI] [PubMed] [Google Scholar]
- 11.Müller, M., R. Schmid, A. Georgopoulos, A. Buxbaum, C. Wasicek, and H. G. Eichler. 1995. Application of microdialysis to clinical pharmacokinetics in humans. Clin. Pharmacol. Ther. 57:371-380. [DOI] [PubMed] [Google Scholar]
- 12.Müller, M., O. Haag, T. Burgdorff, A. Georgopoulos, W. Weninger, B. Jansen, G. Stanek, E. Agneter, H. G. Eichler, and H. Pehamberger. 1996. Characterization of peripheral compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob. Agents Chemother. 40:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller, M., M. Brunner, R. Schmid, E. M. Putz, A. Schmidberger, I. Wallner, and H. G. Eichler. 1998. Comparison of three different experimental methods for the assessment of peripheral pharmacokinetics in humans. Life Sci. 62:PL227-PL234. [DOI] [PubMed] [Google Scholar]
- 14.Müller, M., M. Brunner, U. Hollenstein, C. Joukhadar, R. Schmid, E. Minar, H. Ehringer, and H. G. Eichler. 1999. Penetration of ciprofloxacin into the interstitial space of inflamed foot lesions in non-insulin-dependent diabetes mellitus patients. Antimicrob. Agents Chemother. 43:2056-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paladino, A. 1999. Pharmacoeconomics of antimicrobial therapy. Am. J. Health-System Pharm. 56(Suppl. 3):S25-S28. [DOI] [PubMed] [Google Scholar]
- 16.Smith, A., A. Weber, R. Pandher, J. Williams-Warren, M. L. Cohen, and B. Ramsey. 1997. Utilization of salivary concentrations of ciprofloxacin in subjects with cystic fibrosis. Infection 25:106-108. [DOI] [PubMed] [Google Scholar]
- 17.Stahle, L., P. Arner, and U. Ungerstedt. 1991. Drug distribution studies with microdialysis. III. Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 49:1853-1858. [DOI] [PubMed] [Google Scholar]
- 18.Thomson, C. 1999. The global epidemiology of resistance to ciprofloxacin and the changing nature of antibiotic resistance: a 10 year perspective. J. Antimicrob. Chemother. 43(Suppl. A):31-40. [DOI] [PubMed] [Google Scholar]
- 19.Tulkens, P. M. 1991. Intracellular distribution and activity of antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 10:100-106. [DOI] [PubMed] [Google Scholar]
- 20.Wilson, A. P. R., and R. N. Grüneberg. 1997. Ciprofloxacin: 10 years of clinical experience. Maxim Medical, Magdalen Centre, The Oxford Science Park, Oxford, United Kingdom.
- 21.Zhai, S. Z., X. Wie, B. M. Parker, K. L. Kunze, and R. E. Vestal. 1996. Relation between plasma and saliva concentrations of enoxacin, ciprofloxacin, and theophylline. Ther. Drug Monit. 18:666-671. [DOI] [PubMed] [Google Scholar]