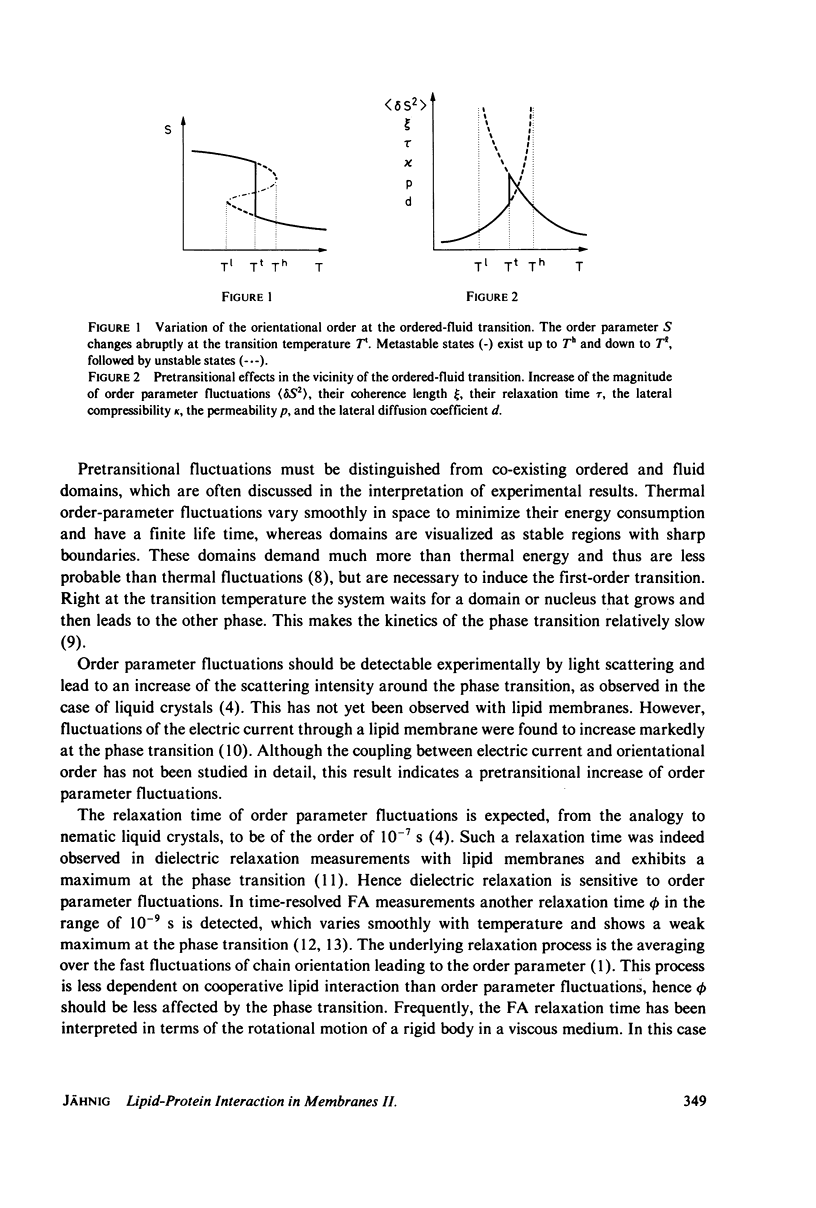
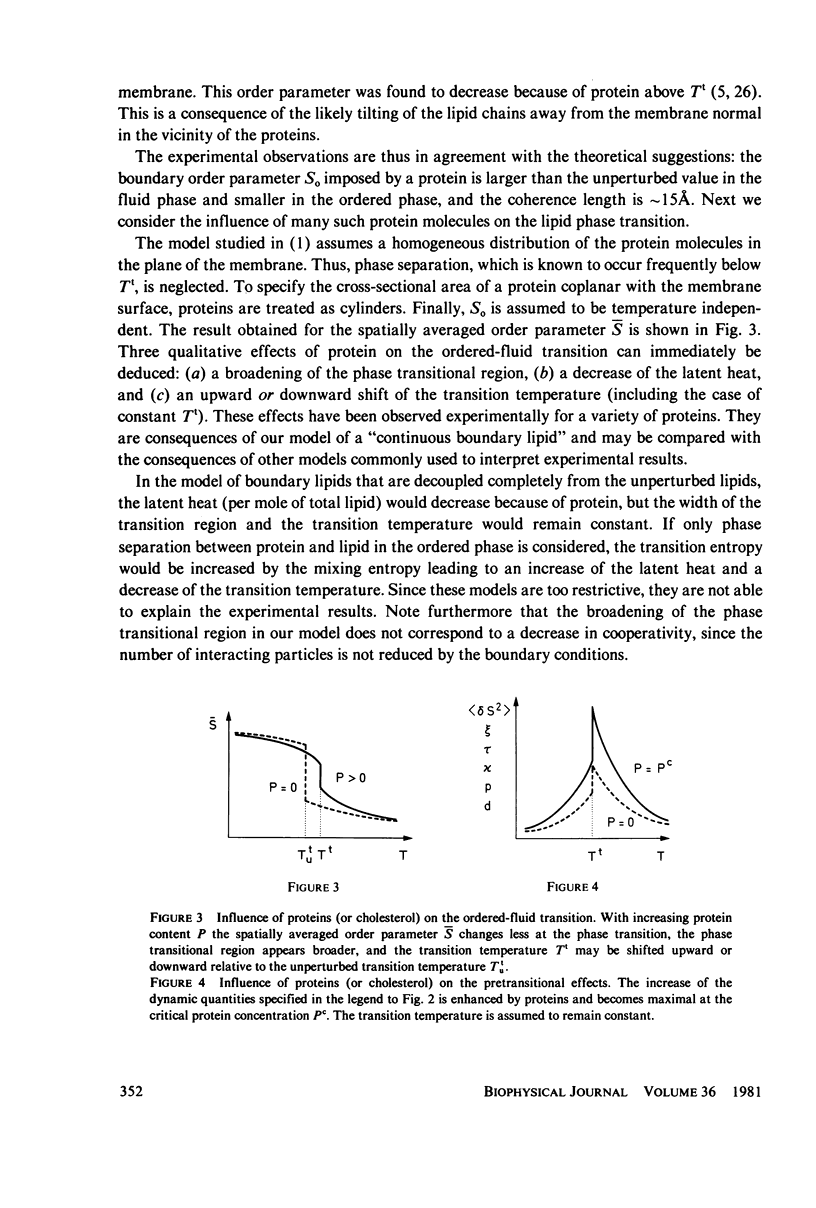
Abstract
The effects arising from lipid-protein and lipid-cholesterol interaction are discussed within the framework of a general theoretical description presented in the preceding paper. Available experimental results are interpreted, and new experiments are proposed. In the fluid lipid phase proteins and cholesterol increase the lipid orientational order in their neighborhood, in the ordered phase they decrease it. This leads to a decrease of the latent heat at the ordered-fluid transition, which vanishes at a critical concentration of protein or cholesterol. Theoretical predictions for the critical concentrations agree with results from calorimetry. The approach to the critical point is accompanied by an increase of thermal fluctuations of the lipid order and an increase of the lipid response on small perturbations. Thus proteins and cholesterol increase the lipid specific heat, lateral compressibility, permeability, and lateral diffusion on both sides of the phase transition. Notions such as decrease of cooperativity or fluidity due to protein or cholesterol are reviewed in this context.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blok M. C., van Deenen L. L., De Gier J. Effect of the gel to liquid crystalline phase transition on the osmotic behaviour of phosphatidylcholine liposomes. Biochim Biophys Acta. 1976 Apr 16;433(1):1–12. doi: 10.1016/0005-2736(76)90172-3. [DOI] [PubMed] [Google Scholar]
- Boheim G., Hanke W., Eibl H. Lipid phase transition in planar bilayer membrane and its effect on carrier- and pore-mediated ion transport. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3403–3407. doi: 10.1073/pnas.77.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatolo W., Sakura J. D., Small D. M., Shipley G. G. Protein-lipid interactions: recombinants of the proteolipid apoprotein of myelin with dimyristoyllecithin. Biochemistry. 1977 May 31;16(11):2313–2319. doi: 10.1021/bi00630a001. [DOI] [PubMed] [Google Scholar]
- Curatolo W., Verma S. P., Sakura J. D., Small D. M., Shipley G. G., Wallach D. F. Structural effects of myelin proteolipid apoprotein on phospholipids: a Raman spectroscopic study. Biochemistry. 1978 May 2;17(9):1802–1807. doi: 10.1021/bi00602a035. [DOI] [PubMed] [Google Scholar]
- Davoust J., Schoot B. M., Devaux P. F. Physical modifications of rhodopsin boundary lipids in lecithin-rhodopsin complexes: a spin-label study. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2755–2759. doi: 10.1073/pnas.76.6.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey P. F., Webb W. W. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry. 1978 Jul 25;17(15):3046–3053. doi: 10.1021/bi00608a016. [DOI] [PubMed] [Google Scholar]
- Gomez-Fernandez J. C., Goni F. M., Bach D., Restall C. J., Chapman D. Protein-lipid interaction. Biophysical studies of (Ca2+ + Mg2+)-ATPase reconstituted systems. Biochim Biophys Acta. 1980 Jun 6;598(3):502–516. doi: 10.1016/0005-2736(80)90031-0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Dencher N. A. Lipid--protein interactions in bacteriorhodopsin--dimyristoylphosphatidylcholine vesicles. Biochemistry. 1981 Feb 17;20(4):840–849. doi: 10.1021/bi00507a029. [DOI] [PubMed] [Google Scholar]
- Heyn M. P. Determination of lipid order parameters and rotational correlation times from fluorescence depolarization experiments. FEBS Lett. 1979 Dec 15;108(2):359–364. doi: 10.1016/0014-5793(79)80564-5. [DOI] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H., Capaldi R. A., Vanderkooi G. Evidence for boundary lipid in membranes. Proc Natl Acad Sci U S A. 1973 Feb;70(2):480–484. doi: 10.1073/pnas.70.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Critical effects from lipid-protein interaction in membranes. I. Theoretical description. Biophys J. 1981 Nov;36(2):329–345. doi: 10.1016/S0006-3495(81)84735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. Y., Gutowsky H. S., Hsung J. C., Jacobs R., King T. E., Rice D., Oldfield E. Nuclear magnetic resonance investigation of the cytochrome oxidase--phospholipid interaction: a new model for boundary lipid. Biochemistry. 1979 Jul 24;18(15):3257–3267. doi: 10.1021/bi00582a010. [DOI] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1977 May 31;16(11):2319–2324. doi: 10.1021/bi00630a002. [DOI] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Effect of cholesterol on the molecular motion in the hydrocarbon region of lecithin bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1978 Nov 14;17(23):5026–5031. doi: 10.1021/bi00616a026. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Tecoma E. S., Wolber P. K., Hudson B. S., Wickner W. T., Simoni R. D. Protein-lipid interactions. Studies of the M13 coat protein in dimyristoylphosphatidylcholine vesicles using parinaric acid. Biochemistry. 1979 Dec 25;18(26):5874–5880. doi: 10.1021/bi00593a018. [DOI] [PubMed] [Google Scholar]
- Knowles P. F., Watts A., Marsh D. Spin-label studies of lipid immobilization in dimyristoylphosphatidylcholine-substituted cytochrome oxidase. Biochemistry. 1979 Oct 16;18(21):4480–4487. doi: 10.1021/bi00588a005. [DOI] [PubMed] [Google Scholar]
- Kuo A. L., Wade C. G. Lipid lateral diffusion by pulsed nuclear magnetic resonance. Biochemistry. 1979 May 29;18(11):2300–2308. doi: 10.1021/bi00578a026. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G., Hogen D. Differential polarized phase fluorometric investigations of diphenylhexatriene in lipid bilayers. Quantitation of hindered depolarizing rotations. Biochemistry. 1979 Feb 6;18(3):508–519. doi: 10.1021/bi00570a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Reithmeier R. A., Shoshan V., Campbell K. P., LeBel D., Herrmann T. R., Shamoo A. E. Ion pathways in proteins of the sarcoplasmic reticulum. Ann N Y Acad Sci. 1980;358:138–148. doi: 10.1111/j.1749-6632.1980.tb15392.x. [DOI] [PubMed] [Google Scholar]
- Marcelja S., Wolfe J. Properties of bilayer membranes in the phase transition or phase separation region. Biochim Biophys Acta. 1979 Oct 19;557(1):24–31. doi: 10.1016/0005-2736(79)90086-5. [DOI] [PubMed] [Google Scholar]
- Mitaku S., Ikegami A., Sakanishi A. Ultrasonic studies of lipid bilayer. Phase transition in synthetic phosphatidylcholine liposomes. Biophys Chem. 1978 Sep;8(4):295–304. doi: 10.1016/0301-4622(78)80012-x. [DOI] [PubMed] [Google Scholar]
- Owicki J. C., McConnell H. M. Lateral diffusion in inhomogeneous membranes. Model membranes containing cholesterol. Biophys J. 1980 Jun;30(3):383–397. doi: 10.1016/S0006-3495(80)85103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanishi A., Mitaku S., Ikegami A. Stabilizing effect of cholesterol on phosphatidylcholine vesicles observed by ultrasonic velocity measurement. Biochemistry. 1979 Jun 12;18(12):2636–2642. doi: 10.1021/bi00579a032. [DOI] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Shepherd J. C., Büldt G. The influence of cholesterol on head group mobility in phospholipid membranes. Biochim Biophys Acta. 1979 Nov 16;558(1):41–47. doi: 10.1016/0005-2736(79)90313-4. [DOI] [PubMed] [Google Scholar]
- Strehlow U., Jähnig F. Electrostatic interactions at charged lipid membranes. Kinetics of the electrostatically triggered phase transition. Biochim Biophys Acta. 1981 Mar 6;641(2):301–310. doi: 10.1016/0005-2736(81)90487-9. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Axelrod D. Reduced lateral mobility of a fluorescent lipid probe in cholesterol-depleted erythrocyte membrane. Biochim Biophys Acta. 1980 Mar 27;597(1):155–165. doi: 10.1016/0005-2736(80)90159-5. [DOI] [PubMed] [Google Scholar]
- Tson- T. Y. Transport of 8-anilino-1-naphthalenesulfonate as a probe of the effect of cholesterol on the phospholipid bilayer structures. Biochemistry. 1975 Dec 16;14(25):5415–5417. doi: 10.1021/bi00696a005. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Effect of phase transition on the kinetics of dye transport in phospholipid bilater structures. Biochemistry. 1975 Dec 16;14(25):5409–5414. doi: 10.1021/bi00696a004. [DOI] [PubMed] [Google Scholar]
- Veatch W. R., Stryer L. Effect of cholesterol on the rotational mobility of diphenylhexatriene in liposomes: a nanosecond fluorescence anisotrophy study. J Mol Biol. 1977 Dec 25;117(4):1109–1113. doi: 10.1016/s0022-2836(77)80017-x. [DOI] [PubMed] [Google Scholar]
- van Zoelen E. J., van Dijck P. W., de Kruijff B., Verkleij A. J., van Deenen L. L. Effect of glycophorin incorporation on the physico-chemical properties of phospholipid bilayers. Biochim Biophys Acta. 1978 Dec 4;514(1):9–24. doi: 10.1016/0005-2736(78)90073-1. [DOI] [PubMed] [Google Scholar]


