Abstract
The importance of supplementary imipenem therapy after a single percutaneous abscess drainage puncture was studied in a mouse model of established mixed-infection abscesses. Animals were treated for 3 days with daily dosing regimens of 384 to 1,536 mg/kg of body weight that took into account the short half-life of this antibiotic in mice. Imipenem therapy in conjunction with abscess drainage was significantly better than drainage alone in reducing the Escherichia coli and Bacteroides fragilis counts in the mixed infections. Furthermore, the killing of B. fragilis by the combination of imipenem therapy and abscess drainage was significantly better than that by imipenem treatment alone. The maximum reductions in E. coli and B. fragilis counts were 1.1 and 2.2 log10 CFU/abscess, respectively. In contrast, the in vitro activity of imipenem was significantly better (maximum reduction, ≥6.2 log10 CFU/ml) against mixed cultures of the same strains even when bacterial numbers similar to those found in the abscesses were used. Comparable in vivo activity was achieved only when treatment was started 30 min before inoculation (reduction for both strains, ≥6.1 log10 CFU/abscess), but this killing was significantly diminished if the start of treatment was delayed until ≥12 h after inoculation. Imipenem concentrations in abscess tissue reached levels above the MIC for E. coli for >60% of the dosing interval. Possible reasons for the reduced activity of imipenem in vivo are discussed, and we conclude that standard susceptibility tests overestimate the efficacy of this antibiotic against the organisms present in these abscesses.
The accepted treatment for patients with small intra-abdominal abscesses involves ultrasound- or computed tomography-guided percutaneous abscess drainage followed by an intensive course of antimicrobial therapy (12). However, the importance of supplementary antibiotic therapy and determination of whether it is beneficial to the further eradication of abscesses have not been extensively studied in animal models (11), even though such studies could provide useful information for the clinician. Furthermore, the selection of the dosing regimen of an antibiotic would normally be based on the in vitro susceptibilities of the individual bacterial strains determined with standard (105 CFU/ml) inocula, even though standard MICs may not be predictive of a successful outcome (16).
Previously, using a mouse model of subcutaneous (s.c.) abscesses, we have studied the efficacies of antimicrobials in the treatment of small well-established mixed-infection abscesses (17). Five days of treatment with very high doses of a broad-spectrum quinolone (well into the range of murine toxicity) were necessary to obtain reductions of more than 2 log10 CFU/abscess for both Escherichia coli and Bacteroides fragilis as well as significant reductions in abscess weight and inflammation.
The aim of the present study was to compare the effect of a single percutaneous abscess drainage puncture and imipenem therapy, either alone or in combination, in the treatment of established mixed-infection abscesses and to assess the significance of the timing of the start of treatment on the in vivo activity of imipenem.
(This study was presented, in part, at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 1998 [I. C. Gyssens et al., Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-37, p. 11, 1998].)
MATERIALS AND METHODS
Antibiotics and media.
Imipenem-cilastin, injectable form (TIëNAM) and sodium powder, was supplied by Merck Sharp & Dohme B.V. (Haarlem, The Netherlands). Wilkens-Chalgren (WC) broth, WC agar, eosin methylene blue (EM) agar, brain heart infusion broth, and DST agar were all supplied by Unipath Ltd. (Haarlem, The Netherlands). Columbia blood agar plates were from Becton Dickinson B.V. (Woerden, The Netherlands).
Animals.
Female specific-pathogen-free BALB/c mice (age, 12 to 18 weeks; weight, 20 to 25 g; IFFA Credo, l'Arbresle, France) were used throughout the study. The cecal contents of male specific-pathogen-free Swiss mice (Broekman Institute B.V., Someren, The Netherlands) were used for the production of autoclaved cecal contents (ACC) (18). All animals received water and food ad libitum. The study was approved by the Institutional Animal Care and Use Committee of the Erasmus MC, Rotterdam, The Netherlands.
Bacterial strains.
B. fragilis ATCC 23745 and E. coli ATCC 25922 were used. The strains were first passaged in BALB/c mice by injecting 108 to 109 CFU of overnight cultures intraperitoneally. After 24 to 48 h, strains were recovered from the liver and spleen and cultured on blood plates, and standardized bacterial suspensions were made in brain heart infusion broth containing 20% glycerol and stored at −80°C until they were required. Overnight cultures were obtained by inoculating 30-ml volumes of WC broth with 0.1 ml of the standardized frozen bacterial suspensions and aerobic (E. coli) or anaerobic (B. fragilis) incubation at 37°C for 18 h.
In vitro studies.
The MICs of imipenem were determined by the standard broth microdilution method with inocula of 105 or 108 CFU/ml, as described previously (18). The in vitro activity of the antibiotic against mixed cultures of B. fragilis and E. coli was measured by the anaerobic time-kill technique outlined previously (18). Briefly, twofold increasing concentrations (0.5 to 64 μg/ml) of imipenem were inoculated with diluted overnight cultures of B. fragilis and E. coli to give mixed cultures containing final bacterial concentrations of 108 or 109 CFU/ml for each strain. Cultures were incubated anaerobically at 37°C for 2, 4, 6, 8, or 24 h. Cultures were washed twice in phosphate-buffered saline (PBS), and bacterial counts were determined by making duplicate serial 10-fold dilutions in PBS and plating 20 μl of each dilution onto EM agar (E. coli) or WC agar containing 100 mg of gentamicin per liter (B. fragilis). The plates were incubated at 37°C aerobically for 24 h (EM agar) or anaerobically for 48 h (WC agar). The effect of the antibiotic on the bacterial counts (E) was expressed as the difference, after 24 h of incubation, between the log10 CFU per milliliter in the absence and in the presence of the antibiotic, as described by the equation E = (Emax × C)/(EC50 + C), where Emax is the maximum observed effect, C is the concentration, and EC50 is the concentration at which 50% of the maximum effect is reached. The EC50 was estimated by nonlinear least-squares regression techniques (GraphPad Prism, version 3.0, for Windows; GraphPad Software, San Diego, Calif.).
Mouse model.
The s.c. abscess model described previously (17) was used, but with a modification to the concentration of the ACC in the inoculum. Briefly, both flanks of groups of three mice each were inoculated (s.c.) with 0.25-ml mixtures containing B. fragilis at 107 CFU and E. coli at 105 CFU (diluted overnight cultures of both organisms) and ACC at 8 mg (dry weight) at a ratio of 1:1:2. Two separate abscesses developed at the inoculation sites. At different times after inoculation (12 h to 9 days) the mice were killed by CO2 asphyxiation and the abscesses were dissected, weighed, and homogenized in 1 ml of PBS for 10 s (Pro 200; B.V. Centraal Magazijn, Abcoude, The Netherlands). The bacterial counts of the resulting suspensions were determined as outlined above.
Drainage of abscesses.
Both flanks of groups of three mice each were inoculated as described above, and abscesses were allowed to develop for 6 days. Mice were anaesthetized intraperitoneally with 70 mg of pentobarbital sodium (Sanofi Diagnostics Pasteur BV, Maassluis, The Netherlands) per kg of body weight, and a closed drainage puncture was performed on each abscess with a 14-gauge (2- by 25-mm) needle (Unimed, Lausanne, Switzerland) attached to a 20-ml monoject syringe (Sherwood-Davies & Geck, s'Hertogenbosch, The Netherlands). The needle was inserted s.c. lateral to the spine and gently guided toward the abscess. Light pressure was applied to puncture the abscess, and all removable pus was aspirated. The needle was carefully extracted and the wound was sealed with Histoacryl (Braun Melsungen AG, Melsungen, Germany). Pus samples were weighed and homogenized in 1 ml of PBS, and bacterial counts were determined as described above. To determine the effect of drainage on subsequent abscess progression, the weights of the abscesses and the bacterial counts in the abscesses were determined either immediately after drainage or 1, 2, or 3 days later.
Pharmacokinetic studies.
Single-dose pharmacokinetic studies with 128 mg of imipenem per kg (administered s.c.) were performed with groups of three mice each 4 days after inoculation with B. fragilis-E. coli-ACC. Blood was removed by orbital puncture, and the serum separated after centrifugation was stored at −80°C until it was used for analysis. To reduce the possible degradation of imipenem, the serum was mixed with an equal volume of 0.5 M morpholineethanesulfonic acid (MES) buffer (pH 6) containing 50% ethylene glycol before the mixture was frozen (13). Total abscess tissue and pus samples were also removed, homogenized (as outlined above) in 0.5 ml of MES buffer, and centrifuged at 13,000 × g for 1 min. The resulting supernatants were stored at −80°C until they were used for analysis.
Imipenem concentrations were determined in duplicate by the agar diffusion bioassay. Standard concentrations were prepared in MES buffer containing ethylene glycol, into which twofold increasing imipenem concentrations were spiked. Test samples containing high concentrations of antibiotic were first diluted in MES buffer and were similarly processed. DST agar plates were inoculated with Staphylococcus aureus ATCC 25923, and 8.5-mm wells were cut out. Each well was filled with 100 μl of standard or test sample, and the plates were incubated overnight at 37°C. Zones of inhibition were read to the nearest 0.05 mm with vernier calipers. Imipenem concentrations were calculated by linear regression. The standard curves were linear within the range of 0.1 to 1.6 μg/ml, with range of r2 values of 0.990 to 0.999. Assay variability was determined by obtaining four replicate measurements for samples containing 0.25, 1.25, or 6.25 μg of imipenem per ml in MES buffer (the samples with 6.25 μg/ml were diluted in MES buffer so that the concentration fell within the standard range of measurements). The test was repeated on 3 separate days. There was a good linear correlation between the observed and the expected values (r2 = 0.98). The median within-run coefficient of variation was 6.6% (range, 3.0 to 9.6%), and the median between-run coefficient was 9.1% (range, 6.0 to 9.8%). (All standard concentrations were prepared in MES buffer since the coefficients of variation reported above were similar to those measured with samples prepared in serum or abscess homogenate.)
Pharmacokinetic parameters were determined by using the MW/Pharm computer program package (Mediware, Groningen, The Netherlands) (14) by use of a one-compartment open model. The parameters obtained were used to simulate various dosing regimens and determine the pharmacokinetic properties of each regimen, such as the maximum concentration of drug and the area under the concentration-time curve during multiple dosing regimens.
Antibiotic treatment.
The influence of the timing of the start of antibiotic treatment on the efficacy of imipenem was determined in groups of three to six mice each that were treated with total daily doses of 384 mg/kg (s.c.) given in six doses of 64 mg/kg/every 4 h (q4h) for 3 days. Treatment was started 30 min before inoculation or 12 h, 24 h, 4 days, or 6 days after inoculation. To determine the effects of higher doses and increased dosing frequencies on efficacy, an additional group of mice was treated 4 days after inoculation with daily doses of 1,536 mg of imipenem kg (128 mg/kg/q2h) for 3 days. The treatment was considered efficacious if decreases in abscess weights and bacterial counts compared to those for saline-treated controls analyzed on the same day were detected.
The effect of combining abscess drainage with imipenem therapy was determined with groups of three mice each 6 days after inoculation. Mice were treated s.c. with total daily doses of 384, 768, or 1,536 mg/kg divided into 6 (q4h) or 12 (q2h) doses. A closed drainage puncture was performed as described above on all abscesses 30 min after the first imipenem dose had been administered. The same daily doses were given to additional groups of mice with undrained abscesses. Treatment was continued for 3 days, and abscess weights and bacterial counts were determined. Untreated animals with drained or undrained abscesses received placebo injections of saline for 3 days.
Statistics.
Abscess weights are given as the mean ± standard error of the mean (SEM), and bacterial counts are given as the mean ± SEM number of CFU (or the mean log10 number of CFU) per abscess or total amount of aspirated pus. The effect of the timing of the start of treatment on the efficacy of imipenem compared to the effect of no treatment was analyzed by the Mann-Whitney test. The different dosing regimens, with or without abscess drainage, were analyzed by Dunn's multiple-comparison test. The average reductions in bacterial counts for all regimens were compared by the unpaired t test. A P value (two-sided) <0.05 was considered significant.
RESULTS
In vitro studies.
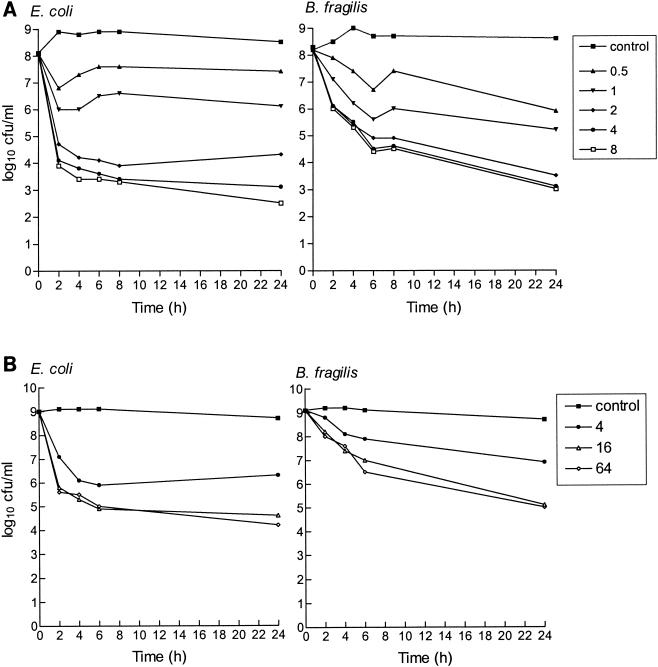
The MICs of imipenem for B. fragilis and E. coli were 0.06 and 0.125 μg/ml, respectively, with an inoculum of 105 CFU/ml and 0.5 and 4 μg/ml, respectively, with an inoculum of 108 CFU/ml. In anaerobic time-kill studies of B. fragilis-E. coli mixed cultures (inoculum, 108 CFU of each organism/ml), imipenem at concentrations ≥2 μg/ml had a bactericidal effect (decrease of >3 log10 CFU/ml compared to the inoculum at the start of the experiment [time zero]) within 2 h for E. coli and within 6 h for B. fragilis (Fig. 1A). The maximum decreases in bacterial counts compared to the counts for control cultures (Emax) after 24 h of incubation were 6.2 and 7.1 log10 CFU/ml for B. fragilis and E. coli, respectively. Similar imipenem concentrations (0.5 and 0.9 μg/ml, respectively) produced 50% of this maximum effect (EC50). When the inoculum was increased to 109 CFU/ml to produce cultures that remained stable throughout the 24 h of incubation (Fig. 1B), the activity of imipenem at concentrations >4 μg/ml was retained, although reduced (maximum bacterial reduction, ≤4.3 log10 CFU/ml), against both bacterial strains.
FIG. 1.
In vitro activity of imipenem against mixed cultures of E. coli ATCC 25922 and B. fragilis ATCC 23745 in an anaerobic time-kill study with an inoculum of 108 or 109 CFU/ml. All imipenem concentrations presented in the key to the symbols are in micrograms per milliliter.
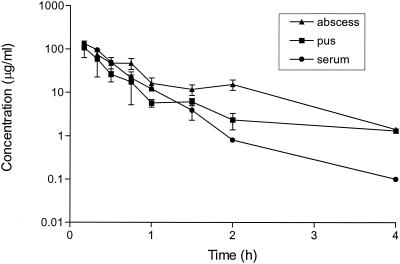
Pharmacokinetic studies.
Imipenem concentrations in serum, total abscess tissue, and aspirated pus were measured after administration of a single s.c. dose of 128 mg/kg (Fig. 2). A peak concentration in serum of 142 μg/ml was reached 0.08 h after administration, and the half-life was 0.27 h. The area under the concentration-time curve from 0 to 24 h was 60.6 mg · h/liter. Similar parameters were obtained after administration of a single s.c. dose of 32 mg of imipenem per kg (data not shown). Accumulation was not observed. Although the imipenem concentrations in total abscess tissue were consistently higher than those in pus, the differences were not significant, thus indicating that the antibiotic had diffused into the core of the abscess. Imipenem concentrations 10 times the MICs for E. coli and B. fragilis (standard inocula) were still present in the abscesses 4 h after injection. Pharmacokinetic studies performed after the administration of multiple doses (12 or 36 doses of 32, 64, or 128 mg/kg) showed no significant antibiotic accumulation in the abscesses (data not shown).
FIG. 2.
Imipenem concentrations in serum, total abscess tissue, and pus samples of mice (three mice per group) treated with a single s.c. injection of 128 mg/kg 4 days after inoculation with B. fragilis ATCC 23745 and E. coli ATCC 25922. Concentrations were measured in a bioassay with S. aureus ATCC 25922 as the test organism. The means ± SEMs are given.
Abscess development and effect of a single drainage puncture.
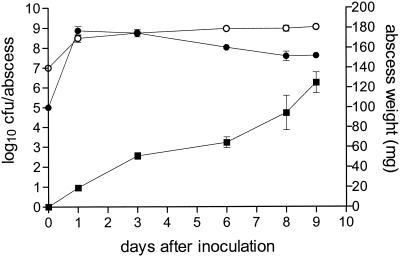
The aim of the present study was to produce abscesses that would be of an appropriate size suitable for drainage studies; therefore, the inoculum contained a higher concentration of ACC (8 mg) than that used in the mouse model reported previously (17). Abscess development was similar (Fig. 3), and even though the average abscess weight was consistently higher than that in the former study (e.g., the average weight on day 6 was 65 ± 5.5 mg in the present study [Fig. 3], whereas it was 54 ± 5.4 mg in the previous study [17]), the differences were not significant. The higher concentration of ACC had no significant effect on the E. coli counts, but there was a significant (P ≤ 0.009) increase in the B. fragilis counts on days 1 to 8, although the difference was never greater than 0.5 log10 CFU/abscess.
FIG. 3.
Development of abscesses in BALB/c mice inoculated with B. fragilis ATCC 23745 (107 CFU) (○) or E. coli ATCC 25922 (105 CFU) (•). ▪, abscess weight. The means ± SEMs of two to three experiments are given.
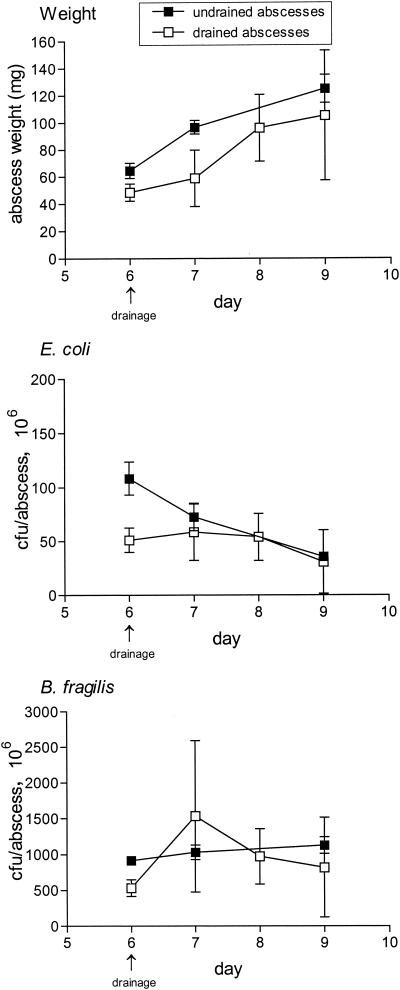
An average of 47.8 ± 4.7 mg of pus containing a total of 5.4 × 107 ± 0.1 (7.7 log10) CFU of E. coli and 3.3 × 108 ± 0.1 (8.5 log10) CFU of B. fragilis could be drained from abscesses 6 days after inoculation (n = 22). Compared to the undrained control abscesses on day 6, drained abscesses had 25 and 50% reductions in abscess weights and bacterial counts, respectively (Fig. 4). If drained abscesses were not treated with antibiotics, growth resumed in the drained abscesses, and after 3 days the abscesses showed a twofold increase in weight that paralleled that of control abscesses. There was a threefold increase in the mean B. fragilis counts within 24 h of drainage, although the increase was not significant compared to the counts for undrained abscesses. The numbers of E. coli remained stable. On day 9, there was no significant difference in bacterial counts between drained and undrained abscesses.
FIG. 4.
Effect of a single percutaneous drainage puncture on abscess development. Abscesses were drained 6 days after inoculation with B. fragilis ATCC 23745 and E. coli ATCC 25922. Abscess weights and bacterial counts were determined immediately after drainage or 1, 2, or 3 days later and compared to those for undrained abscesses for which measurements were obtained on the same days. The means ± SEMs are given.
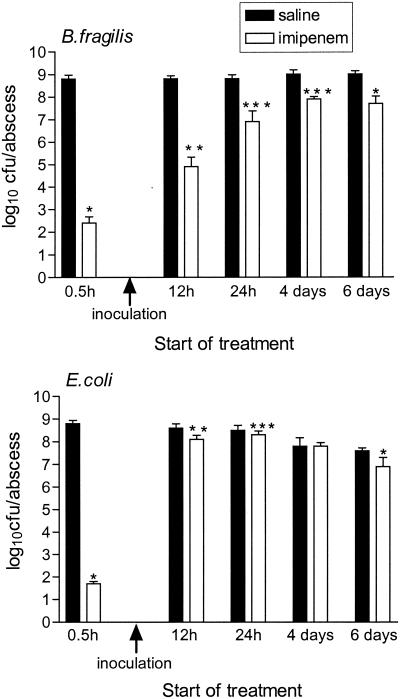
Influence of timing of start of treatment on imipenem efficacy.
Figure 5 depicts the bacterial counts for abscesses treated for 3 days with total daily doses of 384 mg of imipenem per kg (64 mg/kg/q4h). When treatment was started 30 min before inoculation, imipenem was extremely efficient in killing both bacterial strains, with a reduction of ≥6.1 log10 CFU/abscess compared to the counts for the untreated controls processed on the same day. A significant decrease in abscess weights was also found, with an average weight of 25 ± 1.4 mg for treated abscesses compared to an average weight of 48 ± 4.3 mg for the controls (P < 0.001). If the start of treatment was delayed until 12 h after inoculation, a diminished, although significant (P = 0.004), killing of both bacterial strains was still detected, with reductions of 3.9 log10 CFU/abscess for B. fragilis (P = 0.004) and 0.5 log10 CFU/abscess for E. coli. Corresponding reductions in abscess weights were similarly found (35 and 59 mg, respectively; P = 0.03). There was no significant reduction in abscess weights when treatment was started ≥24 h after inoculation; and the reductions in bacterial counts, although mostly significant compared to those for the controls, did not exceed 1.9 log10 CFU/abscess. An increase in daily doses to 1,536 mg/kg, in addition to more frequent dosing (128 mg/kg/q2h) to take into account the short half-life of imipenem in mice, failed to significantly improve the efficacy of imipenem in treating abscesses 4 days after inoculation, with a significant (P < 0.001) decrease in counts of 1.8 log10 CFU/abscess compared to the counts for the controls found only for B. fragilis (data not shown).
FIG. 5.
Influence of timing of start of treatment on efficacy of imipenem. Groups of three mice each were treated with daily doses of 384 mg of imipenem per kg (64 mg/kg/q4h) or saline for 3 days starting 30 min before or 12 h, 24 h, 4 days, or 6 days after inoculation of B. fragilis ATCC 23745 and E. coli ATCC 25922. The means ± SEMs are given. ∗, P ≤ 0.001 compared to saline-treated abscesses; ∗∗, P = 0.004 compared to saline-treated abscesses; ∗∗∗, P ≤ 0.02 compared to saline-treated abscesses.
Effect of abscess drainage on imipenem therapy compared to imipenem therapy alone.
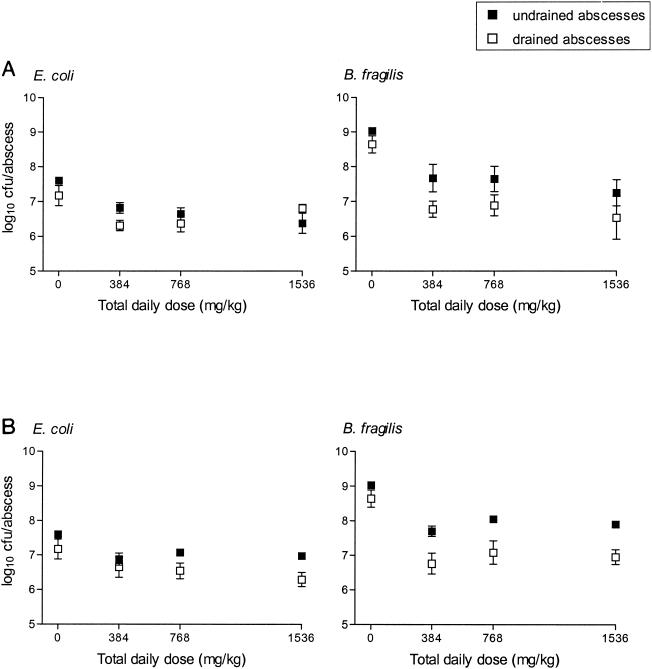
When drained abscesses were additionally treated for 3 days with daily doses of 384 to 1,536 mg of imipenem per kg, there was with most regimens an enhanced killing of both bacterial strains compared to the killing achieved for undrained abscesses treated with the same doses (Fig. 6). These differences, however, were not significant. Compared to the counts in the undrained, untreated abscesses, the drained abscesses had significant (P ≤ 0.05) decreases in the counts of both bacterial strains with all regimens except two (384 mg/kg [64 mg/kg/q4h] and 1,536 mg/kg [128 mg/kg/q2h]). Undrained abscesses treated with the same dosing regimens also had significant (P ≤ 0.05) reductions in the counts of both strains compared to the counts for the controls, but only with doses of 128 mg/kg/q2h. There was no significant reduction in abscess weights and no significant difference between the different dosing regimens for either drained or undrained abscesses. When the average bacterial reductions achieved with all regimens were compared, the killing of B. fragilis was significantly (P < 0.05) better than that of E. coli in both the drained and undrained abscesses, and the overall killing of the anaerobe was significantly (P < 0.001) enhanced when therapy included abscess drainage. Without abscess drainage the reductions in bacterial counts compared to the counts for the undrained control abscesses were 0.8 ± 0.11 and 1.3 ± 0.12 log10 CFU/abscess for E. coli and B. fragilis, respectively. With abscess drainage the reductions were 1.1 ± 0.09 and 2.2 ± 0.08 log10 CFU/ml, respectively (P = 0.053 and P < 0.001, respectively, compared to the results obtained without abscess drainage).
FIG. 6.
Effect of abscess drainage on imipenem therapy compared to effect of imipenem therapy alone. Groups of three mice each were treated 6 days after inoculation of B. fragilis ATCC 23745 and E. coli ATCC 25922 with daily doses of 384, 768, or 1,536 mg of imipenem per kg divided into 6 (q4h) or 12 (q2h) daily doses for 3 days. A single percutaneous drainage puncture was performed on drained abscesses 30 min after the first imipenem dose had been administered, and the bacterial counts were compared with those of undrained abscesses treated with the same daily doses. The means ± SEMs are given.
Pharmacodynamic analysis.
It was not the purpose of the present study to determine the pharmacodynamic index that would best explain the effect of imipenem in this model, and therefore, a comprehensive series of regimens was not used. Nevertheless, since the time above the MIC (T > MIC) is an important index for β-lactams (5, 7), we have calculated the T > MIC for E. coli in both serum and abscesses to correlate this index with imipenem efficacy. With daily doses of 384 mg/kg (64 mg/kg/q4h), the values of T > MIC in serum and abscesses were 63 and 45% of the dosing interval, respectively. However, if the increase in MIC due to the inoculum effect is taken into account, the respective values of the T > MIC would be reduced to 30 and 22% of the interval. With the highest daily dose of 1,536 mg/kg (128 mg/kg/q2h), the values of the T > MIC (inoculum 108 CFU/ml) in serum and abscesses were 73 and 53% of the interval, respectively.
DISCUSSION
With the mouse model of mixed-infection s.c. abscesses described here, we have demonstrated that a single percutaneous abscess drainage puncture is insufficient in reducing abscess bacterial numbers and that the combination of imipenem therapy and abscess drainage is significantly better than imipenem treatment alone for the killing of B. fragilis. Furthermore, we have shown that in vitro susceptibility tests can overestimate the efficacy of imipenem against the organisms in these abscesses.
The persistence of high bacterial counts 3 days after a single drainage puncture illustrates the prerequisite for supplementary antibacterial therapy in the treatment of these established mixed-infection abscesses. Even though 50% of the bacteria could be removed by use of a closed drainage puncture, the bacteria in the abscesses resumed growth, with bacterial counts remaining similar to those found in undrained abscesses 3 days after drainage. Although the increase in the weights of both undrained and drained abscesses was probably due to an ongoing inflammatory response, the host defenses were, presumably, unable to cope with the large numbers of bacteria remaining in the abscesses (8). Since repeated percutaneous drainage punctures could not be performed in this animal model, it should be emphasized that, with the routine practice of continuous catheter drainage procedures (19), the elimination of pus (and bacteria) in the clinical setting would probably be more efficient than was possible in this study with a single puncture. Nevertheless, these results demonstrate the consequences of inadequate bacterial removal.
Our findings corroborate those from the experiments of Lucore et al. (11), who found that surgical drainage alone failed to reduce abscess bacterial numbers in a rabbit model of pyogenic liver abscesses and that antibiotic therapy alone was as effective as antibiotic treatment in conjunction with abscess drainage. Within the individual dosing regimens, we also failed to find a significant difference between the group receiving antibiotic therapy alone and the group receiving antibiotic therapy plus drainage; however, the variations in the amounts of pus removed from individual abscesses complicate the interpretation of the results. Although the greater susceptibility and the lower inoculum effect of B. fragilis compared to those of E. coli would explain the superior killing of the anaerobe by imipenem, it does not clarify the enhanced reduction in B. fragilis counts when therapy included abscess drainage. Nonetheless, the efficacy of imipenem against both strains was modest and was inferior to that of a broad-spectrum quinolone used in a previously published study (17).
In contrast to these in vivo results, the in vitro activity of imipenem was markedly better against mixed cultures of the same E. coli and B. fragilis strains when bacterial numbers similar to those found in the abscesses were used. A similar killing of both strains was found, with a maximum reduction in bacterial counts of ≥6.2 log10 CFU/ml after 24 h of incubation. Comparable in vivo activity was achieved only when treatment was started 30 min before inoculation, i.e., as prophylaxis. If the start of treatment was delayed until 12 h after inoculation, the bacterial killing was considerably decreased and was further impaired when the treatment start time was delayed ≥24 h. These results confirm those in previous reports on reduced antibiotic efficacy caused by progressive delays in the treatment of abscesses (3, 10) and elucidates the previously described substantial reduction in bacterial counts in other animal models when imipenem treatment is started 1 or 12 h after inoculation (4, 15).
There could be several reasons for the decreased efficacy of imipenem in our in vivo model. First, the 12- to 24-h breakpoint after which the treatment of abscesses becomes difficult corresponds to the point when, histologically, the abscess capsule is completely developed (17). This would suggest that the capsule presents a physical barrier to antibiotic penetration that would lead to a reduced antibacterial effect. Although the capsule may influence the diffusion of an antibiotic into the infected site (9), abscesses present additional rigorous environmental conditions that could limit antibiotic activity. For example, the large numbers of bacteria in an inactive metabolic state, the presence of cellular debris and toxic products, as well as the pH of the abscess could all affect the efficacy of an antibiotic in vivo (12).
With regard to antibiotic penetration, imipenem concentrations in abscess tissue reached levels well above the MICs for E. coli and B. fragilis even within the abscess core. However, the pharmacodynamic index that is most predictive of success with β-lactams is T > MIC (5), and maximum efficacy against members of the family Enterobacteriaceae can be achieved when concentrations in serum are above the MIC for at least 60% of the dosing interval (7). From our pharmacodynamic analyses, it would appear that a T > MIC of imipenem in serum of >60% of the dosing interval could also be achieved in this model against E. coli with the lowest doses of 64 mg/kg/q4h. However, if the inoculum effect of E. coli is considered, a comparable T > MIC in serum could be reached only with doses of 128 mg/kg/q2h. Furthermore, the T > MIC of imipenem in abscesses was never more than 53% of the dosing interval.
Nevertheless, if treatment is started early enough, these regimens could be effective against the same mixed infections. Following an initial period of active bacterial growth immediately after inoculation, abscess bacterial counts remain stable for several days (17). The metabolic state of the bacterial strains during treatment, therefore, could also play an important role in the activity of imipenem in vivo. In contrast to other β-lactam antibiotics (6), the activity of imipenem in vitro was retained against metabolically inactive mixed cultures in this study. However, higher concentrations were required and the killing of both strains was reduced compared to the killing of strains in actively growing cultures. However, the threefold increase in B. fragilis numbers within 24 h of abscess drainage and the consequent change in metabolic state could also explain the superior killing of the anaerobe in the drained abscesses.
Although the activity of imipenem can be impaired by a decrease in environmental pH (20), a pH of 6.9 was measured in the center of the abscesses in the present study (unpublished results). Similarly, the low level of protein binding (20%) reported for imipenem (21) would also exclude serum binding as a reason for the reduced in vivo activity. However, reduced bacterial killing in vivo could also be the result of inhibited neutrophil function within the abscesses (1) and the inability of the antibiotic to reach bacteria that had been phagocytosed by these neutrophils (2).
We conclude that imipenem therapy, in conjunction with abscess drainage, is significantly better than drainage alone in reducing the numbers of E. coli and B. fragilis organisms in established mixed-infection abscesses. Furthermore, the killing of B. fragilis by the combination of imipenem therapy and abscess drainage is significantly better than that by imipenem treatment alone. Imipenem diffuses readily into the abscesses, but due to the inoculum effect of the antibiotic, the T > MIC for standard inocula overestimates the effect of imipenem on these abscesses.
Acknowledgments
This study was financially supported by Merck Sharp & Dohme, West Point, Pa.
REFERENCES
- 1.Bamberger, D. M., and B. L. Herndon. 1990. Bactericidal killing of neutrophils in rabbits with experimental acute and chronic abscesses. J. Infect. Dis. 162:186-192. [DOI] [PubMed] [Google Scholar]
- 2.Bamberger, D. M., B. L. Herndon, M. Dew, R. P. Chern, H. Mitchel, L. E. Summers, R. F. Marcus, S. C. Kim, and P. R. Suvarna. 1997. Efficacies of ofloxacin, rifampin, and clindamycin in treatment of Staphylococcus aureus abscesses and correlation with results of an in vitro assay of intracellular bacterial killing. Antimicrob. Agents Chemother. 41:1178-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., M. Dezfulian, and K. Joiner. 1983. Relative efficacy and critical interval of antimicrobial agents in experimental infections involving Bacteroides fragilis. Arch. Surg. 118:181-184. [DOI] [PubMed] [Google Scholar]
- 4.Brook, I. 1989. In vitro susceptibility and in vivo efficacy of antimicrobials in the treament of Bacteroides fragilis-Escherichia coli infection in mice. J. Infect. Dis. 160:651-656. [DOI] [PubMed] [Google Scholar]
- 5.Carbon, C. 1991. Impact of the antibiotic dosage schedule on efficacy in experimental endocarditis. Scand. J. Infect. Dis. 74:163-172. [PubMed] [Google Scholar]
- 6.Cozens, R. M., E. Tuomanen, W. Tosch, O. Zak, J. Suter, and A. Tomasz. 1986. Evaluation of the bactericidal activity of β-lactam antibiotics on slowly growing bacteria cultured in the chemostat. Antimicrob. Agents Chemother. 29:797-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig, W. A. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89-96. [DOI] [PubMed] [Google Scholar]
- 8.Finlay-Jones, J. J., K. V. L. Davies, L. P. Sturm, P. A. Kenny, and P. H. Hart. 1999. Inflammatory processes in a murine model of intra-abdominal abscess formation. J. Leukoc. Biol. 66:583-587. [DOI] [PubMed] [Google Scholar]
- 9.Galandiuk, S., J. Lamos, W. Montgomery, S. Young, and H. C. Polk. 1995. Antibiotic penetration of experimental intra-abdominal abscesses. Am. Surg. 61:521-525. [PubMed] [Google Scholar]
- 10.Joiner, K., B. Lowe, J. Dzink, and J. G. Bartlett. 1982. Comparative efficacy of 10 antimicrobial agents in experimental infections with Bacteroides fragilis. J. Infect. Dis. 145:561-568. [DOI] [PubMed] [Google Scholar]
- 11.Lucore, C. L., M. I. McDonald, M. Wharton, and D. T. Durack. 1986. Experimental anaerobic liver abscess: observations on treatment. Liver 6:125-132. [DOI] [PubMed] [Google Scholar]
- 12.McClean, K. L., G. J. Sheehan, and G. K. M. Harding. 1994. Intraabdominal infection: a review. Clin. Infect. Dis. 19:100-116. [DOI] [PubMed] [Google Scholar]
- 13.Myers, C. M., and J. L. Blumer. 1984. Determination of imipenem and cilastine in serum by high-pressure liquid chromatography. Antimicrob. Agents Chemother. 26:78-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proost, J. M., and D. K. F. Meijer. MW/PHARM, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Mediware, Groningen, The Netherlands. [DOI] [PubMed]
- 15.Rosman, C., J. G. Westerveld, K. Kooi, and R. P. Bleichrodt. 1999. Local treatment of generalised peritonitis in rats; effects on bacteria, endotoxin and mortality. Eur. J. Surg. 165:1072-1079. [DOI] [PubMed] [Google Scholar]
- 16.Soriano, F., P. Garcia-Corbeira, C. Ponte, R. Fernandez-Roblas, and I. Gadea. 1996. Correlation of pharmacodynamic parameters of five β-lactam antibiotics with therapeutic efficacies in an animal model. Antimicrob. Agents Chemother. 40:2686-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stearne, L. E. T., I. C. Gyssens, W. H. Goessens, J. W. Mouton, W. J. Oyen, J. W. van der Meer, and H. A. Verbrugh. 2001. In vivo efficacy of trovafloxacin against Bacteroides fragilis in mixed infections with either Escherichia coli or a vancomycin-resistant strain of Enterococcus faecium in an established-abscess murine model. Antimicrob. Agents Chemother. 45:1394-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stearne, L. E. T., C. Kooi, W. H. Goessens, I. A. J. M. Bakker-Woudenberg, and I. C. Gyssens. 2001. In vitro activity of trovafloxacin against Bacteroides fragilis in mixed culture with either Escherichia coli or a vancomycin-resistant strain of Enterococcus faecium using an anaerobic time-kill technique. Antimicrob. Agents Chemother. 45:243-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Sonnenberg, E., G. R. Wittich, B. W. Goodacre, G. Casola, and H. B. D'Agostino. 2001. Percutaneous abscess drainage: update. World J. Surg. 25:362-372. [DOI] [PubMed] [Google Scholar]
- 20.Winstanley, T. G. 1991. Effect of pH on antibiotics used to treat anaerobic infection. J. Antimicrob. Chemother. 29:594-595. [DOI] [PubMed] [Google Scholar]
- 21.Wise, R., J. M. Andrews, and N. Patel. 1981. N-Formimidoyl-thienamycin a novel beta-lactam: an in-vitro comparison with other beta-lactam antibiotics. J. Antimicrob. Chemother. 7:521-529. [DOI] [PubMed] [Google Scholar]