Abstract
Peptides that exert antimicrobial activity in artificial media may lack activity within blood or other complex biological matrices. To facilitate the evaluation of antimicrobial peptides for possible therapeutic utility, an ex vivo assay was developed to assess the extent and durability of peptide antimicrobial activities in complex fluid biomatrices of whole blood, plasma, and serum compared with those in conventional media. Novel antimicrobial peptides (RP-1 and RP-11) were designed based in part on platelet microbicidal proteins. RP-1, RP-11, or gentamicin was introduced into biomatrices either coincident with, or 2 h prior to, inoculation with an Escherichia coli target organism. Antimicrobial activities of peptides were assessed by quantitative culture 2 h after bacterial inoculation and compared to those of peptide-free and gentamicin controls. In whole blood and homologous plasma or serum, introduction of RP-1 or RP-11 coincident with E. coli was associated with a significant reduction in CFU per milliliter versus the respective peptide-free controls. Moreover, substantial antimicrobial activity remained when RP-1 or RP-11 was placed into whole blood or plasma 2 h prior to E. coli inoculation. These results suggest that the peptides were not rapidly inactivated within these biomatrices. Peptide antimicrobial activities were negatively affected by preincubation in serum or in heat-inactivated serum, compared with those of the respective controls. Peptides RP-1 and RP-11 were consistently effective at lower concentrations in biomatrices than in artificial media, indicating favorable antimicrobial interactions with components of blood or blood fractions. Collectively, these findings support the concept that synthetic peptides can be designed to exert potent antimicrobial activities in relevant and complex biological matrices.
Antimicrobial peptides have been isolated and characterized from virtually every organism surveyed to date, ranging from prokaryotes to humans. Among the most thoroughly studied of these molecules are those from mammalian leukocyte granules and the epithelial mucosa (6, 25). We hypothesize that, for such peptides, localization to these sites likely contributes to optimal antimicrobial functions and minimization of host toxicity by limiting their distribution to these restricted environments. Antimicrobial peptides from these or other sources often exhibit strong antimicrobial activities when tested in artificial media or austere buffer systems in vitro. However, in complex fluid matrices such as whole blood, plasma, and serum, they tend to have relatively poor activity or target selectivity and thus are typically rapidly inactivated or are cytotoxic and/or erythrolytic.
The potential interactions between a given antimicrobial peptide and blood components are multifactorial. For example, whole blood and other fluid biomatrices may contain binding or blocking proteins that may inactivate such a peptide. Similarly, blood may contain peptidases which degrade the peptide over time. Alternatively, blood and blood fractions may contain components that potentiate antimicrobial peptide actions against target pathogens or that are enhanced by antimicrobial peptides, resulting in amplified pathogen killing. Understanding such factors is of direct relevance to the potential therapeutic applications of antimicrobial peptides.
Recent studies have demonstrated that human and other mammalian platelets contain a group of potent antimicrobial peptides, termed platelet microbicidal proteins (PMPs) and thrombin-induced PMPs (tPMPs) (5, 22-24). In comparison to leukocytes and the epithelial mucosa, mammalian platelets respond to microorganisms or soluble mediators of inflammation by liberating the majority of their PMPs and tPMPs directly into the bloodstream. Antimicrobial polypeptides from platelets have some structural and functional similarities with peptides from leukocyte and epithelial sources: PMPs and tPMPs are relatively small (e.g., <15 kDa), and some have a net cationic charge associated with antimicrobial activity and appear to interact with microbial cytoplasmic membranes, followed by intracellular targeting to achieve their ultimate mechanism of action (19, 22, 23). Despite their potent and broad-spectrum antimicrobial activities, these peptides have relatively limited cytotoxicity for relevant mammalian cells (e.g., vascular endothelial cells), as assessed by preliminary studies in vitro (M. R. Yeaman, A. S. Ibrahim, J. A. Ritchie, S. G. Filler, A. S. Bayer, J. E. Edwards, Jr., and M. A. Ghannoum, IDSA Annu. Meet., abstr. 491, 1995). Thus, PMPs and tPMPs likely possess structure-activity relationships optimally suited for antimicrobial functions without concomitant host cytotoxicity in complex biomatrices such as whole blood, plasma, and serum (8, 23, 24).
Based in part on these concepts, the present investigation tested novel synthetic peptides intended to exert optimal antimicrobial functions in relevant settings, including blood and blood fractions. An ex vivo screening assay was developed to define potential antimicrobial peptide-biomatrix interactions by using a conventional antibiotic, gentamicin, as a comparator. The results demonstrate that the test peptides exert potent and remarkably durable antimicrobial activity in whole blood and homologous plasma when coincubated with serum compared to conventional artificial media.
MATERIALS AND METHODS
Antimicrobial agents.
RP-1 is an 18-amino-acid synthetic peptide designed to incorporate structure-activity attributes characteristic of PMPs and tPMPs (23). In this respect, RP-1 (N-ALYKK5FKKKL10LKSLK15RLG-C; mass, 2,162.8 kDa) has some structural similarities with the antimicrobial peptide C18G, previously described by Darveau et al. (1). However, RP-1 is structurally distinct, as it contains a signature dipeptide motif, believed to influence antimicrobial activity. RP-11 is a novel 13-amino-acid synthetic peptide (N-ALYKR5LFKKL10KKF-C; mass, 1,683.1 kDa) also designed to reflect structure-function attributes of PMPs and tPMPs. RP-1 and related peptides have been previously demonstrated to exert antimicrobial activities in a chemically defined medium by radial diffusion assay in vitro (M. R. Yeaman, M. D. Joshi, K. D. Gank, D. Kahler, and W. H. Welch, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-170, 1999).
For the present study, peptides were made with a Symphony multiplex synthesizer (Rainin, Woburn, Mass.) located in the Biopolymer Synthesis Core Facility at Harbor-UCLA Research and Education Institute (Torrance, Calif.). Standard Nα-9-fluorenylmethoxy carbonyl chemistry with appropriate R-protecting groups was used in coupling on polyethylene Wang resin (American Peptide Company, Sunnyvale, Calif.), and 1-hydroxybenzotriazole activation was used to optimize coupling efficiency. The resulting peptides were completed, cleaved, and deprotected by routine methods. The cleaved peptide was then precipitated, washed, and lyophilized. Following this process, the crude peptide was dissolved in 0.1% acetic acid (pH 5.5). Samples were then subjected to analytical and preparative reversed-phase high-pressure liquid chromatography (RP-HPLC) and matrix-assisted laser desorption ionization-time of flight mass spectroscopy (UCLA Protein Microsequencing Facility) to confirm homogeneous purification and verify correct mass, respectively. Efficiency of solid-phase synthesis of RP-1 and RP-11 was typically ≥75%, and RP-HPLC allowed rapid baseline purification of each of the peptides, which were confirmed to have masses indicative of correct synthesis. Purified antimicrobial peptides or gentamicin sulfate (Fujisawa USA Inc., Deerfield, Ill.) was suspended and diluted in sterile phosphate-buffered saline (PBS; pH 5.5 or 7.2 for the respective assay conditions) prior to use.
Microorganism.
The well-characterized Escherichia coli strain ML-35 was used as the target organism in these studies (7). In pilot experiments (data not shown), this strain was confirmed to be susceptible to gentamicin: the gentamicin MIC and minimum bactericidal concentration (MBC) for the strain in standard broth microdilution assays in accordance with National Committee for Clinical Laboratory Standards guidelines were each ≤1 μg/ml (12). In addition, this strain was resistant to serum to a degree sufficient to permit survival to be assessed in the absence of antimicrobials, thus allowing for its use in the development of the biomatrix assay (see below). For assays described below, the organism was cultured to mid-logarithmic phase in brain-heart infusion broth (Difco Laboratories, Detroit, Mich.) at 37°C with agitation, harvested by centrifugation, washed, and resuspended in PBS (Irvine Scientific, Santa Ana, Calif.; pH 7.2) and the inoculum was determined by spectrophotometry and validated by quantitative culture (see below).
Antimicrobial assay in biomatrices.
The antimicrobial activities of RP-1, RP-11, and gentamicin in human whole blood and homologous plasma and serum fractions were assessed. For a given experiment, 30 ml of fresh whole blood was obtained from a healthy volunteer in compliance with institutional policies and practice for informed consent. Citrate anticoagulant was used to anticoagulate two-thirds (20 ml) of the whole-blood sample (final concentrations, 16.8 μM sodium citrate and 4.2 μM citric acid) (19, 22). Six milliliters of the citrated whole blood was set aside, and the other 14 ml was subjected to low-speed centrifugation (210 × g), yielding a platelet-rich plasma supernatant. These preparations comprised the whole-blood and plasma biomatrices, respectively. The remaining 10 ml of nonanticoagulated whole blood was allowed to spontaneously clot in Vacutainer tubes (red top; Becton Dickinson, Franklin Lakes, N.J.; 1 h) and centrifuged (300 × g) to yield homologous serum (“homologous” refers to a blood fraction, in this case serum, derived from the same donor whose whole blood was also directly studied). In selected experiments, antimicrobial peptides and gentamicin were compared for activities in pooled normal human serum (PNHS; Gemini Scientific, Woodland, Calif.) and heat-inactivated PNHS (56°C for 30 min; ΔPNHS). Routinely, samples at dilutions of between 10−2 and 10−5 were quantitatively cultured in parallel from each condition at the 0 and 2 h time points. Because sonication of whole blood or plasma risked liberating leukocyte and platelet antimicrobial peptides, samples were incubated with continuous agitation and vortexed immediately prior to quantitative culture. These methods achieved reproducible results, with typical standard deviations of ≤10% within and across individual experiments, despite day-to-day variations in blood donors. Additionally, these approaches provided a concurrent comparison of antimicrobial effects and corroboration of CFU and established a reliable limit of sensitivity of 1 log10 CFU/ml in test biomatrices and media. Thus, apparent counts of less than log10 1.0 CFU/ml were conservatively interpreted as being log10 1.0 CFU/ml when calculating log survival. This approach likely underestimated the true antimicrobial activity of study peptides. For purposes of comparison in the present study, concentrations of RP-1, RP-11, or gentamicin yielding 50 or 90% reductions in CFU per milliliter were considered 50 or 90% inhibitory concentrations (IC50 or IC90, respectively) and concentrations yielding no detectable surviving E. coli were considered MBC.
For assays, whole blood, plasma, homologous serum, PNHS, and ΔPNHS were distributed in 85-μl aliquots into 96-well microtiter plates (Corning Glass Works, Corning, N.Y.). Two strategies were employed to compare the extent and durability of antimicrobial activity of RP-1, RP-11, and gentamicin in these biological matrices. In one series of experiments, peptide or gentamicin (in 5-μl volumes) was introduced into each matrix simultaneously with pathogen inoculation (10 μl), yielding a final inoculum of 105 CFU/ml and a final range in RP-1, RP-11, and gentamicin concentrations of 1 to 50 μg/ml in a 100-μl total volume (of which 85% represented the fluid matrix of interest). In parallel experiments performed concurrently, RP-1, RP-11, or gentamicin was introduced into each matrix prior to pathogen inoculation, incubated with constant agitation for 2 h at 37°C, and then inoculated with the organism. In either strategy, pathogen inoculation and exposure to antimicrobial agent initiated an additional incubation period of 2 h at 37°C with agitation. At the end of the 2-h exposure period, mixtures were vortexed and diluted in PBS to ensure dispersion and quantitatively cultured in triplicate onto blood agar as outlined above. Colonies representative of surviving organisms were enumerated and calculated in units of CFU per milliliter after a 24-h incubation of blood agar plates at 37°C.
Antimicrobial assay in conventional media.
For comparison, the antimicrobial activities of RP-1, RP-11, and gentamicin were tested in a conventional-medium system employing cation-supplemented Mueller-Hinton II broth (MHB; Becton Dickinson, Sparks, Mo.) as appropriate in accordance with National Committee for Clinical Laboratory Standards guidelines (11). Assays performed with conventional media were conducted in parallel with biomatrix assays. Because whole blood and blood fractions, as well as sites of inflammation or serum generation, may undergo a shift in pH, the efficacies of the study antimicrobial agents were compared in MHB adjusted to pH 5.5 or 7.2. As described for the biomatrix assay, the peptide was introduced into the media either 2 h prior to, or simultaneously with, the pathogen inoculum; the introduction of the peptide was followed by a 2-h exposure period. At the end of the 2-h exposure period, reaction mixtures were processed for quantitative culture as described above. As in the biomatrix assays, the limit of detection in conventional media assays was considered 1 log10 CFU/ml.
Data analysis.
Experiments were performed a minimum of two independent times on different days and with different blood donor sources. Two-way analysis of variance was used to compare differences in antimicrobial agent activities in distinct test conditions (K.Yoshioka, KyPlot, version 2.0 beta, public domain, http://www.qualest.co.jp, 2000). The Bonferroni correction for multiple comparisons was used where appropriate. P values ≤0.05 were considered significant.
RESULTS
The antimicrobial activities of RP-1, RP-11, and gentamicin in whole blood and homologous plasma and serum fractions, as well as in conventional media at pH 5.5 and 7.2, were simultaneously compared. In selected experiments, antimicrobial agent efficacies in PNHS or ΔPNHS were compared. The pHs of whole blood, homologous plasma, and homologous serum were 7.3, 7.7, and 6.6, respectively, prior to preincubation and 7.2, 7.8, and 6.6, respectively, following 2 h of incubation.
Antimicrobial activities of RP-1, RP-11, and gentamicin coincubated with the organism. (i) Efficacy in whole blood.
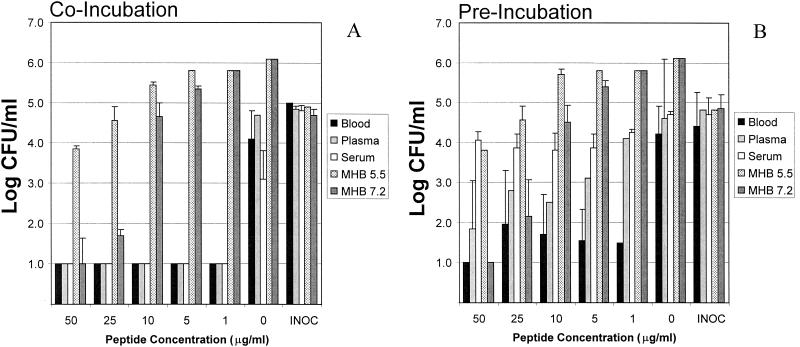
In whole blood, an inoculum of log10 5 CFU/ml yielded a mean end point in E. coli viability of log10 4.1 CFU/ml (Fig. 1A) after a 2-h incubation at 37°C. This reduction of −Δlog10 0.9 CFU/ml was attributed to the inherent antimicrobial activity of whole blood. The comparative antimicrobial effects of peptides and gentamicin were normalized to the same biomatrix in the absence of an antimicrobial agent. By comparison, in whole blood containing RP-1 at concentrations ranging from 50 to 1 μg/ml, no viable E. coli cells were detectable after a 2-h incubation. The presence of as little as 1 μg of RP-1 per ml was associated with a mean reduction of ≥log10 3.1 CFU/ml in whole blood upon coincubation with organisms for 2 h at 37°C. The MBC of RP-1 in whole blood (MBCblood) was ≤1 μg/ml (Table 1). In comparison, viable E. coli cells were not detectable in whole blood containing RP-11 at concentrations ranging from 5 to 50 μg/ml but were present (log10 1 CFU/ml) in whole blood containing RP-11 at 1 μg/ml (Fig. 2C). Thus, the MBCblood of RP-11 was 5 μg/ml (Table 1). Similar to what was found with RP-1, E. coli was undetectable in whole blood containing gentamicin at concentrations ranging from 1 to 50 μg/ml. Therefore, gentamicin exhibited an MBCblood of ≤1 μg/ml versus E. coli (Table 1). Vehicle alone caused no significant reduction in E. coli viability in whole blood compared with controls.
FIG. 1.
Comparative efficacies of RP-1 versus E. coli ML-35 in biomatrices or in MHB at pH 5.5 and 7.2. (A) Coincubation of the peptide and organism simultaneously added to the test biomatrix or medium; (B) preincubation of the peptide in the biomatrix or medium for 2 h at 37°C prior to introduction of the organism. The E. coli inoculum (INOC) was 105 CFU/ml, and the threshold of sensitivity was considered 1 log10 CFU/ml.
TABLE 1.
Comparative efficacies of antimicrobial peptides RP-1 and RP-11 and gentamicin in fluid biomatrices and artificial media under specified assay conditionsa
| Biomatrix or medium and peptide or drug | IC or MBC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| Coincubation
|
2-h preincubation
|
|||||
| IC50 | IC90 | MBC | IC50 | IC90 | MBC | |
| Biomatrices | ||||||
| Whole blood | ||||||
| RP-1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | 50 |
| RP-11 | ≤1 | ≤1 | 5 | ≤1 | 50 | >50 |
| Gentamicin | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
| Plasma | ||||||
| RP-1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤5 | >50 |
| RP-11 | ≤1 | ≤1 | >50 | ≤1 | >25 | >50 |
| Gentamicin | 10 | 10 | 25 | 10 | 10 | 25 |
| Homologous serum | ||||||
| RP-1 | ≤1 | ≤1 | ≤1 | ≤1 | >50 | >50 |
| RP-11 | ≤5 | ≤5 | 50 | >50 | >50 | >50 |
| Gentamicin | ≤1 | ≤1 | ≤1 | ≤1 | 5 | 5 |
| PNHS | ||||||
| RP-1 | ≤1 | ≤5 | >50 | ≤1 | ≤5 | >50 |
| RP-11 | 25 | >50 | >50 | >50 | >50 | >50 |
| Gentamicin | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
| ΔPNHS | ||||||
| RP-1 | 50 | >50 | >50 | >50 | >50 | >50 |
| RP-11 | 50 | >50 | >50 | >50 | >50 | >50 |
| Gentamicin | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
| Conventional media | ||||||
| MHB at pH 5.5 | ||||||
| RP-1 | 5 | ≤25 | >50 | 10 | ≤25 | >50 |
| RP-11 | >50 | >50 | >50 | >50 | >50 | >50 |
| Gentamicin | 5 | 5 | >50 | 5 | 5 | >50 |
| MHB at pH 7.2 | ||||||
| RP-1 | ≤5 | ≤10 | 50 | ≤5 | ≤10 | 50 |
| RP-11 | >50 | >50 | >50 | >50 | >50 | >50 |
| Gentamicin | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 | ≤1 |
See Materials and Methods for specific conditions of assay.
FIG. 2.
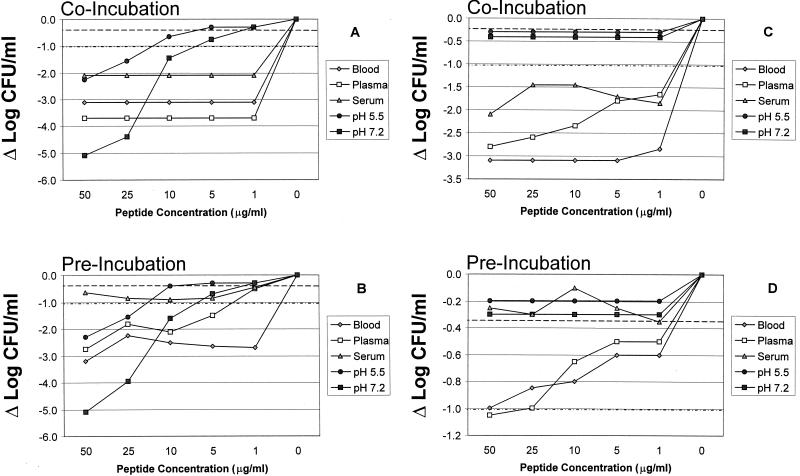
(A and B) Comparative impact of RP-1 on survival of E. coli ML-35 when coincubated with the organism (A) or preincubated for 2 h at 37°C prior to introduction of the organism into test biomatrices or MHB (pH 5.5 or 7.2) (B). (C and D) Comparative impact of RP-11 on survival of E. coli ML-35 when RP-11 was coincubated with the organism (C) or preincubated for 2 h at 37°C prior to introduction of the organism into biomatrices or MHB (pH 5.5 or 7.2) (D). The E. coli inoculum was 105 CFU/ml, and the threshold of sensitivity was considered 1 log10 CFU/ml. Data are normalized to the respective controls. Each point represents the mean of two independent experiments with ≤10% standard error. Error bars are intentionally omitted for clarity. Note the change in scale of the ordinate for the different panels. Dashed and dot-dashed lines indicate logs of survival corresponding to the respective IC50 and IC90 levels, allowing estimation of peptide concentrations translating to these inhibitory concentrations (see also Table 2).
(ii) Efficacy in homologous plasma.
In homologous plasma, a log10 5 CFU/ml inoculum yielded a mean end point E. coli viability of log10 4.7 CFU/ml after a 2-h incubation at 37°C (Fig. 1A). This change of −Δlog10 0.3 CFU/ml was not significantly different from control, suggesting that plasma had minimal inherent anti-E. coli activity in this assay. However, no viable E. coli cells were detectable in plasma containing RP-1 at concentrations ranging from 50 to 1 μg/ml when the peptide and organism were introduced simultaneously and incubated for 2 h at 37°C (Fig. 1A). Therefore, RP-1 exhibited an assay MBCplasma of ≤1 μg/ml (Table 1), corresponding to a mean reduction of ≥log10 3.7 CFU/ml versus E. coli in this biomatrix. By comparison, RP-11 exerted considerably less anti-E. coli activity in plasma than did RP-1. For example, RP-11 displayed dose-dependent efficacy in plasma, with surviving log10 CFU/ml of 1.4, 1.6, 1.9, 2.9, and 3.1 in the presence of RP-11 at 50, 25, 10, 5, and 1 μg/ml, respectively. However, since viable E. coli cells were detectable in plasma containing RP-11 at concentrations as high as 50 μg/ml, the apparent RP-11 MBCplasma was interpreted to be ≥50 μg/ml (Table 1). The efficacy of gentamicin in plasma against E. coli was significantly lower than that of RP-1 (P < 0.05 for concentrations of 10 μg/ml or less; Table 1). For example, exposure to gentamicin at 50 or 25 μg/ml in plasma yielded no detectable E. coli, while 10 μg/ml caused a mean reduction of −Δlog10 2.1 CFU/ml compared with controls (P < 0.05 versus the respective controls). However, 1 or 5 μg of gentamicin/ml in plasma failed to cause a significant reduction in E. coli viability compared to controls. From these data, gentamicin MBCplasma was observed to be 25 μg/ml (Table 1). Vehicle alone caused no significant reduction in E. coli viability in plasma compared with controls.
(iii) Efficacy in homologous serum.
In fresh homologous human serum, an inoculum of log10 5 CFU/ml yielded a mean E. coli survival of log10 3.1 CFU/ml (Fig. 1A) after a 2-h incubation at 37°C. This reduction of −Δlog10 1.9 CFU/ml was attributed to the intrinsic antimicrobial activity of serum. As with whole blood, the comparative antimicrobial effects of peptides and gentamicin in serum were normalized to the serum biomatrix lacking an antimicrobial agent. Coincubation in serum containing RP-1 at concentrations ranging from 50 to 1 μg/ml yielded no viable E. coli cells detectable after a 2-h incubation. As little as 1 μg of RP-1 per ml of serum introduced simultaneously with E. coli was associated with a reduction of ≥log10 2.1 CFU/ml, translating to an RP-1 MBCserum of ≤1 μg/ml (Table 1). Likewise, RP-11 at 50 μg/ml of serum yielded no detectable E. coli survival; however, RP-11 concentrations of 25 μg/ml or less failed to render E. coli undetectable in serum. Therefore, the RP-11 MBCserum under these assay conditions was interpreted as 50 μg/ml (Table 1). No surviving E. coli cells were detectable in serum containing gentamicin across the 50- to 1-μg/ml range tested. These results indicate an apparent gentamicin MBCserum of ≤1 μg/ml versus E. coli as defined in this assay (Table 1). As with the whole-blood and plasma assays above, vehicle alone caused no significant reduction in E. coli viability in serum compared with controls.
(iv) Efficacy in MHB at pH 5.5.
In MHB at pH 5.5, an E. coli inoculum of log10 5 CFU/ml produced a mean cell density of log10 6.1 CFU/ml after a 2-h incubation at 37°C (Fig. 1A). The comparative antimicrobial effects of peptides and gentamicin were normalized to this increase in log10 CFU/ml of 1.1 in this medium. Under this condition, RP-1 exerted a significant and dose-dependent anti-E. coli effect at concentrations of 50 and 25 μg/ml (P < 0.05 versus controls) but no significant difference from controls at 10, 5, or 1 μg/ml (Fig. 1A). These findings correspond to RP-1 MBCMHB5.5, IC90, MHB5.5, and IC50, MHB5.5 values of >50, 50, and 25 μg/ml, respectively (Table 1). RP-11 exhibited no anti-E. coli activity in MHB at pH 5.5 at the concentrations tested, yielding MBC, IC90, and IC50 values ≥50 μg/ml under these conditions (Table 1). In contrast, gentamicin concentrations as low as 5 μg/ml were associated with no detectable E. coli recoverable from this medium, while 1 μg/ml achieved no reduction compared with controls. These data correspond to gentamicin MBCMHB5.5, IC90, MHB5.5, and IC50, MHB5.5 values of 5 μg/ml each (Table 1).
(v) Efficacy in MHB at pH 7.2.
In conventional medium at pH 7.2, an E. coli inoculum of log10 5 CFU/ml produced a mean cell density of log10 6.1 CFU/ml after a 2-h incubation at 37°C (Fig. 1A). As above, the comparative antimicrobial effects of peptides and gentamicin were normalized to an increase in log10 CFU/ml of 1.1 in this medium in their absence. In these conditions, RP-1 at 50 μg/ml yielded no detectable surviving E. coli, mean survivals of log10 1.7 and log10 4.7 CFU/ml at 25 and 10 μg/ml (P < 0.05 versus controls), respectively, and no reduction compared with controls at either 5 or 1 μg/ml (Fig. 1A). These findings yield RP-1 MBCMHB7.2, IC90, MHB7.2, and IC50, MHB7.2 values of 50, 10, and 5 μg/ml, respectively (Table 1). It is notable that RP-1 exerted greater antimicrobial activity in MHB at pH 7.2 than at pH 5.5 in preincubation studies (P < 0.05; Table 1). By comparison, RP-11 exhibited no anti-E. coli activity in MHB at pH 7.2, yielding MBCMHB7.2, and IC90, MHB7.2, and IC50, MHB7.2 concentrations of ≥50 μg/ml (Table 1). In contrast, a gentamicin concentration as low as 1 μg/ml yielded no detectable E. coli after the 2-h incubation period, corresponding to MBCMHB7.2, IC90, MHB7.2, and IC50, MHB7.2 values all ≤1 μg/ml by definition in this assay system (Table 1).
Antimicrobial activities of RP-1, RP-11, and gentamicin following 2-h preincubation. (i) Efficacy following preincubation in whole blood.
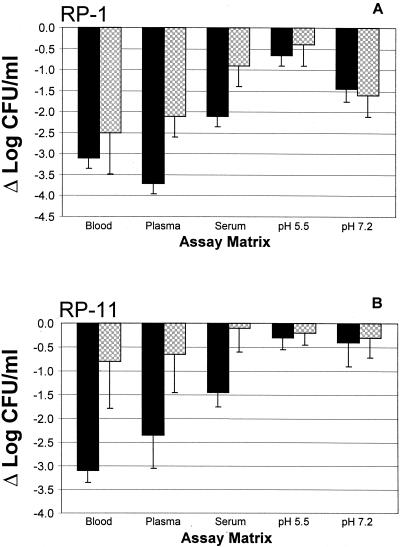
Consistent with the results above, an inoculum of log10 5 CFU/ml in whole blood preincubated for 2 h at 37°C yielded a mean end point in E. coli viability of log10 4.2 CFU/ml (Fig. 1B). Thus, preincubation had no significant effect on the intrinsic anti-E. coli activity of whole blood. The antimicrobial effects of RP-1, RP-11, and gentamicin were normalized to those of the respective controls in this matrix. After preincubation in whole blood, the apparent potency of RP-1 was modestly, but not significantly, reduced compared with that found during coincubation (P > 0.05; Fig. 1B, 2A and B, and 3A). For example, in the preincubation assay, only RP-1 at a concentration of 50 μg/ml yielded undetectable E. coli; lower concentrations achieved strong and equivalent antimicrobial activities but not complete killing of the organism (Fig. 1B and Table 1). The MBCblood, IC90, blood, and IC50, blood values reflect the comparative reduction in antimicrobial activity of RP-1 due to preincubation versus coincubation (Tables 1 and 2; Fig. 2 and 3). Of note, preincubation of RP-11 in whole blood resulted in significantly less antimicrobial activity than coincubation of the peptide and organism (P < 0.05; Fig. 2C and D and 3B). Reductions in log10 CFU/ml ranged from 1.0 at 50 μg/ml to 0.6 at 1 μg/ml, yielding increased MBCblood and ICblood values for RP-11 when it was preincubated in whole blood compared with those when it was coincubated (Fig. 2D and 3B; Table 1). In contrast, across the test range from 50 to 1 μg/ml, gentamicin reduced the amount of viable E. coli to levels below the threshold of detection even after a 2-h preincubation in whole blood (Fig. 2C and D and 3B; Table 1). As before, vehicle alone caused no significant reduction in E. coli viability in preincubated whole blood compared with controls.
TABLE 2.
IC50 and IC90 of RP-1 and RP-11 versus E. coli ML-35 in test biomatrices and MHB estimated from co- and preincubation assay resultsa
| Biomatrix or medium | Conditionb | IC50 (μg/ml) | IC90 (μg/ml) |
|---|---|---|---|
| RP-1c | |||
| Whole blood | Co | ≤1 | ≤1 |
| Pre | ≤1 | ≤1 | |
| Homologous plasma | Co | ≤1 | ≤1 |
| Pre | ≤1 | 3 | |
| Homologous serum | Co | ≤1 | ≤1 |
| Pre | ≤1 | >50 | |
| MHB at pH 5.5 | Co | 6 | 15 |
| Pre | 10 | 18 | |
| MHB at pH 7.2 | Co | 2 | 6 |
| Pre | 3 | 7 | |
| RP-11d | |||
| Whole blood | Co | ≤1 | ≤1 |
| Pre | ≤1 | 50 | |
| Homologous plasma | Co | ≤1 | ≤1 |
| Pre | ≤1 | 30 | |
| Homologous serum | Co | ≤1 | ≤1 |
| Pre | ≥50 | ≥50 | |
| MHB at 5.5 | Co | ≥50 | ≥50 |
| Pre | ≥50 | ≥50 | |
| MHB at 7.2 | Co | ≥50 | ≥50 |
| Pre | ≥50 | ≥50 |
FIG. 3.
Comparative influences of coincubation (solid bars) versus preincubation (cross-hatched bars) on the antimicrobial efficacies of RP-1 (A) and RP-11 (B) in biomatrices or MHB (pH 5.5 or 7.2). These data illustrate the effects of these antimicrobial peptides at a concentration of 10 μg/ml. Data are normalized to the respective controls.
(ii) Efficacy following preincubation in homologous plasma.
Similar to results from coincubation studies, inoculation of log10 5 CFU of E. coli/ml into plasma preincubated for 2 h at 37°C yielded a mean end point viability of log10 4.6 CFU/ml (Fig. 1B) following a 2-h incubation postinoculation. This change of −Δlog10 0.4 CFU/ml compared with control was not significant and suggested that the limited anti-E. coli activities of coincubated and preincubated plasma were equivalent. RP-1 and RP-11 maintained anti-E. coli activity after a 2-h incubation in homologous plasma. As observed in whole blood, higher peptide concentrations were required to achieve levels of killing in plasma following preincubation equivalent to those following coincubation (Fig. 2 and 3). For example, RP-1 under this condition exerted considerable antimicrobial activity but did not completely sterilize E. coli even at a concentration of 50 μg/ml (Fig. 1B, 2B, and 3A). These effects translated to corresponding increases in preincubation RP-1 MBCplasma and ICplasma values compared to coincubation results (Tables 1 and 2). In contrast, RP-11 exerted significantly reduced anti-E. coli bioactivity in plasma following preincubation compared to that when it was introduced simultaneously with the organism (P < 0.05 versus coincubation; Fig. 2D and 3B; Tables 1 and 2). Consistent with its activity in plasma coincubation experiments, gentamicin at concentrations of 50 or 25 μg/ml preincubated with plasma yielded undetectable E. coli, while 10 μg/ml caused a mean reduction of log10 2.0 CFU/ml compared with the control, but 5 or 1 μg/ml failed to reduce E. coli viability. Therefore, the preincubation MBCplasma and ICplasma values for gentamicin were not different from those seen with coincubation (P > 0.05; Table 1). Vehicle alone caused no significant reduction in E. coli viability in preincubated plasma versus controls.
(iii) Efficacy following preincubation in homologous serum.
An E. coli inoculum of log10 5 CFU/ml yielded a mean survival of log10 4.7 CFU/ml (Fig. 1B) in serum preincubated for 2 h at 37°C. Thus, preincubated serum did not cause a significant reduction in CFU per milliliter compared with the inhibitory effect of coincubated serum (P > 0.05; see above). However, preincubation of RP-1 in homologous serum resulted in a dramatic loss of antimicrobial activity compared with coincubation in this biomatrix (P < 0.05; Fig. 2A and B; Tables 1 and 2). Similarly, RP-11 failed to achieve significant reductions in E. coli CFU following preincubation at any test concentration in serum compared with control (P > 0.05 versus control; Fig. 2C and D; Table 1), in contrast to its antimicrobial activity in coincubation experiments (P < 0.05 versus coincubation). Gentamicin at concentrations ranging from 50 to 5 μg/ml retained its efficacy against E. coli following preincubation in serum but failed to significantly reduce organism viability when tested at 1 μg/ml. These data yielded gentamicin MBCserum and IC90, serum values that were generally 1 dilution greater than those observed in serum with coincubation (Table 1). As before, vehicle alone produced no significant reduction in E. coli viability in preincubated serum compared with controls.
(iv) Efficacy in preincubated MHB at pH 5.5 or 7.2.
Anti-E. coli activities of RP-1, RP-11, and gentamicin following preincubation in artificial medium at pH 5.5 or 7.2 were not significantly different from those observed in the same media with coincubation (Fig. 1 to 3 and Table 1). As in the coincubation studies (above), the IC90 and MBC for RP-1 in MHB were modestly lower at pH 7.2 than at pH 5.5 (P < 0.05 at concentrations of 50 and 25 μg/ml; Fig. 1; Table 1)
Influence of serum status on antimicrobial efficacy.
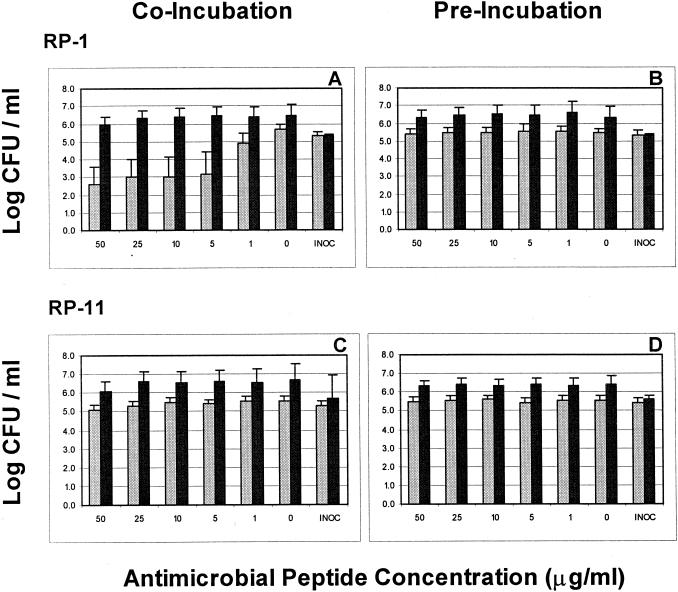
The results observed above suggested that components present in blood or blood fractions influenced the antimicrobial activities of RP-1 and RP-11 but had comparably less impact on the anti-E. coli activity of gentamicin. Moreover, the observation that peptide antimicrobial activities were most negatively affected by preincubation in serum suggested that they may be influenced by complement, peptidases, or other heat-labile factors in this biomatrix. Therefore, the efficacies of RP-1, RP-11, and gentamicin in PNHS and ΔPNHS with and without preincubation for 2 h were assessed as described above (Fig. 4). PNHS consistently achieved a somewhat greater killing of E. coli than ΔPNHS (Fig. 4); this was attributed to the respective intrinsic antimicrobial characteristics of these matrices. Similar to homologous serum (above), RP-1 at concentrations of 50 to 5 μg/ml exerted significant anti-E. coli activity when introduced into PNHS simultaneously with the organism (Fig. 4A). However, this effect of RP-1 was absent on coincubation in ΔPNHS or in PNHS or ΔPNHS following a 2-h preincubation prior to organism inoculation (Fig. 4B; Table 1). In PNHS, RP-11 exerted modest antimicrobial activity only at concentrations of 50 and 25 μg/ml (Fig. 4C). Corresponding RP-11 IC90plasma and MBCplasma values for PNHS differed somewhat from those for fresh homologous serum (Table 1). In contrast, no significant antimicrobial activities against E. coli were observed when RP-11 was preincubated in PNHS or ΔPNHS for 2 h prior to introduction of the organism (Fig. 4D; Table 1). Consistent with results observed in fresh homologous serum, the anti-E. coli activities of gentamicin in PNHS and ΔPNHS prior to and following preincubation were equivalent (Table 1).
FIG. 4.
Comparative efficacies of RP-1 (A and B) and RP-11 (C and D) versus E. coli ML-35 in coincubation (A and C) and preincubation (B and D) conditions in PNHS (light bars) and ΔPNHS (dark bars). As in other experiments, the E. coli inoculum (INOC) was 105 CFU/ml and the threshold of sensitivity was considered 1 log10 CFU/ml.
DISCUSSION
An ex vivo assay was developed to provide a relevant challenge to the extent and duration of antimicrobial peptide efficacy in complex biomatrices including whole blood, plasma, and serum. Plasma differs from whole blood in that the former is essentially devoid of leukocytes and erythrocytes. Moreover, serum is differentiated from either whole blood or plasma by being a cell-free biomatrix generated following the coagulation of whole blood and plasma components, with associated activation of proteases and other factors. The activities of two antimicrobial peptides were compared with that of gentamicin in parallel in each of these matrices and in conventional artificial media at pH 5.5 or 7.2. The assay design included simultaneous introduction of the antimicrobial agent and organism into biomatrices or media or a 2-h preincubation of the antimicrobial agents in test matrices or media prior to inoculation of organisms. In conventional antimicrobial assays, gram-negative organisms may exhibit a “regrowth” phenomenon following exposure to cationic agents such as aminoglycoside antibiotics (20), obscuring the differentiation of bacteriostatic from bactericidal effects. Awareness of this potential effect resulted in a biomatrix assay that integrated a quantitative culture over a 24-h period prior to colony enumeration to verify bactericidal effect. Thus, the present assay was developed to screen for the extent and durability of antimicrobial activity of peptides in complex biological matrices. In addition, the activities of the antimicrobial peptides in PNHS and ΔPNHS were examined to assess the influence of heat-labile components on their efficacy. The assay system described differs from other methods that focus on whole blood (18) and that do not include the preincubation step integral to the assay developed for the present investigation.
Several important observations emerged from these investigations. RP-1 exerted anti-E. coli activity in conventional media; this activity was modestly enhanced at pH 7.2 compared to that at pH 5.5 and was retained following a 2-h preincubation in media. Importantly, RP-1 exhibited potent antimicrobial activities in whole blood and homologous plasma or serum. In some conditions tested, RP-1 exerted anti-E. coli effects that were equivalent to, or exceeded, those of gentamicin. Additionally, the antimicrobial activities of RP-1 in the biological matrices were generally greater than those in conventional media. In contrast to RP-1 and peptides structurally related to RP-11, which demonstrate robust antimicrobial activities in nutrient broth and radial diffusion assays in vitro (Yeaman et al., 38th ICAAC), RP-11 exerted essentially no anti-E. coli activity in MHB at either pH 5.5 or 7.2. Nonetheless, as intended based on its derivation from PMP and tPMP structural themes (i.e., antimicrobial peptides active in the cardiovascular milieu), RP-11 exhibited striking antimicrobial activity in whole blood, plasma, and serum, and this activity endured in whole blood and plasma following a 2-h preincubation prior to organism inoculation. These observations suggest that RP-11 is inhibited in the relatively complex MHB medium, has activity that is amplified by components in biomatrices, or potentiates these components to exert antimicrobial activity. The reduced antimicrobial effect of gentamicin at pH 5.5 versus pH 7.2 is consistent with previous studies regarding pH optima for aminoglycoside antibiotics (2, 10, 15, 20).
Modification of the biomatrix, through either changes to its constituents (e.g., whole blood versus serum) or preincubation with a peptide, may affect antimicrobial peptide activity in a variety of ways. As noted above, the biomatrix may directly bind the peptide, leading to inactivation. The necessary binding components may be present in some of the matrices but not others; for example, binding to erythrocytes would occur only in whole blood. Alternatively, biomatrices may contain peptidases or other reactants that degrade the peptide in a time-dependent fashion. Such degradation would contribute to loss of activity in the 2-h-preincubation assay compared to that in the assay involving immediate coincubation of the antimicrobial peptide and target organism. The activation of plasma components during the generation of serum may also yield peptidases not present in native whole blood or plasma and may contribute to any loss of activity observed during serum preincubation compared with the activity in plasma. Alternatively, biomatrices may contribute components that potentiate peptide antimicrobial activity. Thus, both RP-1 and RP-11 appear to be more potent in blood and blood fractions than in artificial media. However, RP-11 was considerably more sensitive than RP-1 to inhibition or inactivation due to preincubation in biomatrices. For example, activation of blood to generate serum or preincubation in serum may eliminate positive cooperative factors or effects that enhance the net bactericidal activities of antimicrobial peptides, resulting in an apparent decrease in their efficacy. Given the distinct structures of RP-1 and RP-11, these results may provide insights into structure-activity relationships governing the fate of antimicrobial peptides such as RP-1 and RP-11 in complex biological settings such as blood or blood fractions.
Screening for activity in biological matrices is an important step in identifying antimicrobial peptides with potential as systemic therapeutic agents. Based on experiences with RP-1 and RP-11, care in the selection of an appropriate matrix for, and in interpreting the results of, antimicrobial assays evaluating such agents must be exercised. In this regard, fresh whole blood or blood fractions may represent particularly relevant matrices for evaluation of potential systemic therapeutic antimicrobial peptides. For example, RP-1 and RP-11 antimicrobial activities in such matrices appeared to be potentiated compared with those in conventional test media. As anticipated, blood itself exerted a modest degree of intrinsic antimicrobial activity against E. coli. Thus, one or more blood components may interact with the peptides or this target organism to enhance killing. Such potentiation has been observed with other conventional antibiotics (3, 9, 14) and tPMPs in vitro (4). Likewise, studies by Yan and Hancock (21) implicated synergistic interactions of antimicrobial peptides and lysozyme in vitro. Varra et al. suggested that an outer membrane-disorganizing peptide sensitized E. coli to the bactericidal activity of serum in vitro, hypothetically by deshielding the organism to the effects of complement fixation (16). Subsequently, this same group demonstrated that the polymyxin B nonapeptide (PMBN) acted synergistically with fresh human serum to effect bactericidal activity against gram-negative bacteria in vitro (17). It has also been suggested that complement contributes to the potentiation of other antimicrobial peptides (1). Importantly, the present findings indicate that RP-1 does not require complement to demonstrate antimicrobial activity, as evidenced in the defined-medium experiments. In contrast, neither RP-1 nor RP-11 demonstrated antimicrobial activity in heat-inactivated serum (ΔPNHS). Consistent with these results, Oh et al. (12) found that short peptides consisting of l-amino acids lost antimicrobial activity in heat-inactivated serum. Additionally, low-density lipoprotein has been implicated as an inhibitor of small-alpha-helical-peptide activity in serum (13). It may also be hypothesized that heat inactivates potentiating factors in serum, but the loss of RP-1 antimicrobial activity in this matrix implies influences beyond this effect. As RP-1 is a potent antimicrobial agent in conventional media, its lack of efficacy in ΔPNHS suggests that a blocking or inhibitory factor(s) is generated during heat inactivation of serum. Ostensibly, this effect could result from one or more simple binding reactants or specific inhibitors of the antimicrobial action(s) of RP-1 and RP-11.
The above considerations emphasize the validity and importance of assays which evaluate the antimicrobial activities of peptides under rigorous conditions potentially relevant to their development to function as therapeutic agents in vivo. The assay described was designed and developed to provide a relevant assessment of the degree and duration of peptide antimicrobial activities in complex biological matrices. However, the differences between this assay and conventional assays should be understood. For example, even in preincubation assays, the antimicrobial agent and target organism are subsequently incubated for 2 h prior to quantitative culture. Substantial inactivation of the antimicrobial agent may occur over this period, leading to false-negative results (e.g., apparent lack of antimicrobial activity of a peptide possessing intrinsic activity). The potential for this effect can be minimized if shortened exposure of the organism (e.g., shortened incubation periods) are found to be sufficient to detect efficacy. Additionally, the biological matrices themselves (e.g., whole blood and serum) may exhibit differential antimicrobial effects, making quantitative expression of the antimicrobial activity of the agent of interest more difficult. For example, antimicrobial activities of the synthetic peptides in fresh homologous human serum were greater than those in frozen PNHS. The present study utilized killing in the presence of the antimicrobial agent, compared to killing by the matrix itself, to best represent the additional effects of the agent of interest. Differential potencies in distinct biomatrices or conventional media with differing pHs may reflect influences of pH on intrinsic structure-activity relationships of antimicrobial peptides such as RP-1 and RP-11. These influences may be significant in specific contexts in which peptides may exert optimal antimicrobial activities in vivo.
Acknowledgments
We are grateful to Alan Waring, codirector of the Biopolymer Synthesis Core Facility of the Harbor-UCLA Research and Education Institute, for his expertise in solid phase peptide synthesis and quality control. We thank William Welch (University of Nevada, Reno) for providing helpful comments.
These studies were supported by grants from the National Institutes of Health (AI48031 and RR14857 to M.R.Y. and AI39108 to A.S.B).
REFERENCES
- 1.Darveau, R. P., J. Blake, C. L. Seachord, W. L. Cosand, M. D. Cunningham, L. Cassiano-Clough, and G. Maloney. 1992. Peptides related to the carboxyl terminus of human platelet factor IV with antibacterial activity. J. Clin. Investig. 90:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuursted, K. 1997. Postexposure factors influencing the duration of postantibiotic effect: significance of temperature, pH, cations, and oxygen tension. Antimicrob Agents Chemother 41:1693-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hostacka, A. 1998. Serum sensitivity and cell surface hydrophobicity of Klebsiella pneumoniae treated with gentamicin, tobramycin, and amikacin. J. Basic Microbiol. 38:383-388. [PubMed] [Google Scholar]
- 4.Koo, S. P., M. R. Yeaman, and A. S. Bayer. 1996. Staphylocidal action of thrombin-induced platelet microbicidal protein is influenced by microenvironment and target cell growth phase. Infect. Immun 64:3758-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krijgsveld, J., S. A. Zaat, J. Meeldijk, P. A. van Veelen, G. Fang, B. Poolman, E. Brandt, J. E. Ehlert, A. J. Kuijpers, G. H. Engbers, J. Feijen, and J. Dankert. 2000. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J. Biol. Chem. 275:20374-20381. [DOI] [PubMed] [Google Scholar]
- 6.Lehrer, R. I., and T. Ganz. 2002. Defensins of vertebrate animals. Curr. Opin. Immunol. 14:96-102. [DOI] [PubMed] [Google Scholar]
- 7.Lehrer, R. I., A. Barton, K. A. Daher, S. S. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Investig. 84:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy, O. 2000. Antimicrobial proteins and peptides of blood: templates for novel antimicrobial agents. Blood 96:2664-2672. [PubMed] [Google Scholar]
- 9.Miglioli, P. A., U. Schoffel, and L. Gianfranceschi. 1996. The in vitro synergistic inhibitory effect of human amniotic fluid and gentamicin on growth of Escherichia coli. Chemotherapy 42:206-209. [DOI] [PubMed] [Google Scholar]
- 10.Oh, J. E., S. Y. Hong, and K. H. Lee. 1999. Structure-activity relationship study: short antimicrobial peptides. J. Pept. Res. 53:41-46. [DOI] [PubMed] [Google Scholar]
- 11.Nanavaty, J., J. E. Mortensen, and T. R. Shryock. 1998. The effects of environmental conditions on the in vitro activity of selected antimicrobial agents against Escherichia coli. Curr. Microbiol. 36:212-215. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Peck-Miller, K. A., R. P. Darveau, and H. P. Fell. 1993. Identification of serum components that inhibit the tumoricidal activity of amphiphilic alpha helical peptides. Cancer Chemother. Pharmacol. 32:109-115. [DOI] [PubMed] [Google Scholar]
- 14.Pruul, H., and P. J. McDonald. 1992. Potentiation of antibacterial activity of azithromycin and other macrolides by normal human serum. Antimicrob. Agents Chemother. 36:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmen, H. P., H. Battaglia, T. Kossmann, and J. Blaser. 1993. Effect of peritoneal fluid pH on outcome of aminoglycoside treatment of intraabdominal infections. World J. Surg. 17:393-397. [DOI] [PubMed] [Google Scholar]
- 16.Vaara, M., P. Viljanen, T. Vaara, and P. H. Makela. 1984. An outer membrane-disorganizing peptide PMBN sensitizes Escherichia coli to serum bactericidal action. J. Immunol. 132:2582-2589. [PubMed] [Google Scholar]
- 17.Viljanen, P., H. Kayhty, M. Vaara, and T. Vaara. 1986. Susceptibility of gram-negative bacteria to the synergistic bactericidal action of serum and polymyxin B nonapeptide. Can. J. Microbiol. 32:66-69. [DOI] [PubMed] [Google Scholar]
- 18.Wallis, R. S., M. Palaci, S. Vinhas, A. Hise, F. C. Ribeiro, K. Landen, S. H. Cheon, H. Y. Song, M. Phillips, R. Dieteze, and J. J. Ellner. 2001. A whole blood bactericidal assay for tuberculosis. J. Infect. Dis. 183:1300-1303. [DOI] [PubMed] [Google Scholar]
- 19.Xiong, Y. Q., A. S. Bayer, and M. R. Yeaman. 2002. Inhibition of Staphylococcus aureus intracellular macromolecular synthesis by thrombin-induced platelet microbicidal proteins. J. Infect. Dis. 185:348-356. [DOI] [PubMed] [Google Scholar]
- 20.Xiong, Y. Q., J. Caillon, H. Drugeon, G. Potel, and D. Baron. 1996. Influence of pH on adaptive resistance of Pseudomonas aeruginosa to aminoglycosides and their postantibiotic effects. Antimicrob. Agents Chemother. 40:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan, H., and R. E. W. Hancock. 2001. Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob. Agents Chemother. 45:1558-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeaman, M. R., Y. Q. Tang, A. J. Shen, A. S. Bayer, and M. E. Selsted. 1997. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect. Immun. 65:1023-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeaman, M. R. 1997. The role of platelets in antimicrobial host defense. Clin. Infect. Dis. 25:951-970. [DOI] [PubMed] [Google Scholar]
- 24.Yeaman, M. R., and A. S. Bayer. 1999. Antimicrobial peptides from platelets. Drug Resist. Updates 2:116-126. [DOI] [PubMed] [Google Scholar]
- 25.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]