Abstract
Despite the discovery of novel β-lactamases such as extended-spectrum β-lactamases (ESBLs), imported AmpC, and carbapenem-hydrolyzing β-lactamases at least a decade ago, there remains a low level of awareness of their importance and how to detect them. There is a need to increase the levels of awareness of clinical laboratories about the detection of newer β-lactamases. Therefore, a study was conducted in 2000 to investigate the occurrence of these β-lactamases in Klebsiella pneumoniae isolates at 24 U.S. medical centers. To enhance the likelihood of detecting imported AmpC and carbapenem-hydrolyzing β-lactamases, participating laboratories were permitted to include archived strains (1996 to 2000) that were intermediate or resistant to either cefoxitin or imipenem. The β-lactamase production of 408 isolates positive by screening of 1,123 isolates was investigated by ESBL phenotypic confirmation tests; and for AmpC and carbapenem-hydrolyzing β-lactamases, three-dimensional tests, isoelectric focusing, β-lactamase inhibitor studies, spectrophotometric assays, induction assays, and molecular tests were used. ESBL-producing isolates were detected at 18 of the 24 sites (75%), imported AmpC-producing isolates were detected at 10 sites (42%), inducible imported AmpC-producing isolates were detected at 3 sites (12.5%), and a molecular class A carbapenem-hydrolyzing enzyme was detected at 1 site (4%). No class B or D carbapenem-hydrolyzing enzymes were detected. ESBLs and imported AmpC β-lactamases were detected at a significant number of sites, indicating widespread penetration of these enzymes into U.S. medical institutions. Because these enzymes may significantly affect therapeutic outcomes, it is vital that clinical laboratories be aware of them and be able to detect their occurrence.
Extended-spectrum β-lactamases (ESBLs) and plasmid-mediated AmpC β-lactamases are novel β-lactamases of increasing clinical concern. ESBLs are typically plasmid-mediated, clavulanate-susceptible enzymes that hydrolyze penicillins, cephalosporins, and aztreonam. They have been detected worldwide in gram-negative pathogens, principally members of the family Enterobacteriaceae (5, 15, 24, 25, 33, 37). Plasmid-mediated AmpC β-lactamases are derivatives of the chromosomally encoded, clavulanate-resistant AmpC cephalosporinases of Enterobacter cloacae, Citrobacter freundii, Morganella morganii, Hafnia alvei, and other yet-to-be-determined gram-negative bacilli. Like ESBLs, these enzymes have been detected worldwide (3-5, 23). Genes encoding both ESBLs and plasmid-mediated AmpC β-lactamases are usually located on large multidrug resistance plasmids. Therefore, both types of enzymes are typically associated with resistance to multiple antibiotics, leaving few therapeutic options. Other novel β-lactamases of clinical concern include carbapenem-hydrolyzing enzymes of molecular classes A, B, and D (18, 26, 37). Although ESBLs and plasmid-mediated AmpC β-lactamases were reported 19 and 13 years ago, respectively, these enzymes continue to elude the detection capabilities of many clinical laboratories (34, 37). Carbapenem-hydrolyzing enzymes also pose diagnostic challenges.
The major technical difficulties in detecting novel β-lactamases probably arise because the pathogens that produce these enzymes do not always show in vitro resistance to substrate antibiotics or the phenotypes may resemble those associated with other resistance mechanisms. Because routine susceptibility tests do not always indicate clinically relevant resistance, it is important that phenotypes be evaluated by experienced microbiologists (6, 9, 16, 21, 22, 38).
With this in mind, the present study was designed to increase awareness and demonstrate the need to detect and monitor the occurrence of novel β-lactamases such as ESBLs, plasmid-mediated AmpC β-lactamases, and carbapenem-hydrolyzing β-lactamases. The goal was to investigate the occurrence (not prevalence) of these types of β-lactamases in 24 U.S. hospitals and to use the findings to alert health professionals about the need for vigilance and preparedness. Although these enzymes may be produced by a variety of organisms, the study focused only on Klebsiella pneumoniae because this species is the most common ESBL-producing organism and because it lacks a chromosomal AmpC gene, making it an ideal indicator organism for the detection of both ESBLs and plasmid-mediated (or transferable) AmpC β-lactamases (37). For the reasons given below, the AmpC β-lactamases detected in K. pneumoniae isolates in this study are referred to as imported AmpC β-lactamases.
MATERIALS AND METHODS
Isolates.
Up to 50 consecutive, nonduplicate nosocomial isolates of K. pneumoniae were requested from each of 24 U.S. hospitals (see Acknowledgments). To enhance the detection of AmpC and carbapenem-hydrolyzing β-lactamases, laboratories were given the option of including archived isolates (1996 to 2000) that were intermediate or resistant to either cefoxitin or imipenem by NCCLS criteria (20). All isolates were phenotypically screened on-site by microdilution MIC testing, and any isolate meeting any of four criteria were referred to Creighton University for retesting and determination of resistance mechanisms. The four screening criteria were as follows: (i) cefpodoxime, ceftriaxone, ceftazidime, cefotaxime, cefepime, or aztreonam MIC ≥2 μg/ml; (ii) eightfold or greater decrease in the MIC of ceftriaxone or ceftazidime for the isolate when the isolate was tested with the particular agent in combination with 2 μg of clavulanate per ml (i.e., a decrease of 3 doubling dilutions); (iii) a cefoxitin MIC ≥16 μg/ml; or (iv) an imipenem MIC ≥1 μg/ml.
Organism identifications were confirmed where necessary with the API 20E, Vitek, or Vitek 2 system (bioMérieux Inc., Hazelwood, Mo.) in combination with any supplementary tests that were appropriate, such as tests for motility, spot biochemical tests, and 16S rRNA analysis (MIDI, Inc., Newark, Del.).
Susceptibility testing.
Susceptibilities were determined by using custom-made dehydrated microdilution MIC panels containing doubling dilutions of ertapenem, imipenem, cefoxitin, ceftriaxone, ceftriaxone plus a constant concentration of clavulanate (2 μg/ml), ceftazidime, ceftazidime plus a constant concentration of clavulanate (2 μg/ml), cefotaxime, cefepime, cefpodoxime, and piperacillin-tazobactam (Dade MicroScan, Sacramento, Calif.).
β-Lactamase investigations.
ESBL production was confirmed by phenotypic inhibitor-based tests with ceftriaxone and ceftazidime, tested alone and in combination with 2 μg of clavulanate per ml. The criterion for ESBL production was a reduction of the ceftazidime or ceftriaxone MIC by at least eightfold (i.e., at least 3 doubling dilutions) in the presence of clavulanate (36). The criteria for AmpC production were production of an enzyme that was resistant to inhibition by clavulanate but susceptible to inhibition by cloxacillin and that hydrolyzed cefoxitin. AmpC β-lactamases were investigated by using (i) the three-dimensional test with cefoxitin to demonstrate that an isolate for which the cefoxitin MIC was ≥16 μg/ml possessed an enzyme capable of hydrolyzing cefoxitin (35), (ii) isoelectric focusing (IEF) to determine the pI(s) of an isolate's β-lactamase(s), (iii) IEF overlays to investigate the capabilities of clavulanate and cloxacillin to inhibit the β-lactamase(s) produced by the isolate (29), (iv) molecular tests (see below), and (v) tests for inducibility by cefoxitin and/or imipenem. Inducibility by cefoxitin and/or imipenem was tested by the disk approximation method (30) and/or by a broth induction method, in which spectrophotometric assays with crude cell sonicates were used to compare the level of β-lactamase production that had occurred in log-phase growth in the presence and absence of one-quarter the MIC of the inducing agent (31). Carbapenem-hydrolyzing enzyme production was investigated by evaluating the abilities of cell sonicates to hydrolyze ertapenem and imipenem in microbiological and spectrophotometric assays (19, 31) and by the IEF overlay procedure (29). The inhibition characteristics of carbapenem-hydrolyzing enzymes were investigated by spectrophotometric assays with 100 μM imipenem as the substrate and preincubation of 100 μl of the crude enzyme preparation for 10 min at 37°C with 50 μl of each of the following inhibitors: 1.0 M NaCl (inhibitor of class D β-lactamases), 100 M tazobactam (primarily an inhibitor of class A β-lactamases), and 0.25 M EDTA (an inhibitor of class B β-lactamases) (1, 7, 8, 11).
Molecular analyses.
Template DNA preparation and PCR amplifications were carried out as described previously in a final volume of 50 μl with 2 μl of template DNA and 0.5 μM primer (13). The MgCl2 concentration (2.0 mM) and the annealing temperature required are indicated with the appropriate primer set in Table 1. The amplicons generated by the primers specific for blaDHA, blaFOX, blaCMY, or blaKPC-1 were sequenced directly after treatment with ExoSAP-IT, as directed by the manufacturer (U.S. Biochemical Corp., Cleveland, Ohio). All amplicons were generated and sequenced at least twice by automated PCR cycle sequencing with dye-terminator chemistry with a DNA stretch sequencer from Applied Biosystems. The inducible AmpC β-lactamase gene designated blaACT-1-like was amplified by PCR with primers designed from Enterobacter cloacae ampR and blaACT-1 sequences (Table 1).
TABLE 1.
Primers used in this study
| Targeta | Primerb | Sequencec | Annealing temp (°C)d | Size (bp)c | nt positionf | GenBank accession no. |
|---|---|---|---|---|---|---|
| DHA | DHA-1F | CCGTTACTCACACACGGAAGG | 50 | 1,199 | 1619-1639 | AF055067 |
| DHA | DHA-1R | CGTATCCGCAGGGGCCTGTTC | 2819-2799 | AF055067 | ||
| KPC-2 | KPC1F | GCTACACCTAGCTCCACCTTC | 46 | 990 | 92-112 | AF297554 |
| KPC-2 | KPC1R | GACAGTGGTTGGTAATCCATGC | 1081-1060 | AF297554 | ||
| FOX | FOXUP1 | CACCACGAGAATAACC | 46 | 1,180 | 683-698 | X77455 |
| FOX | FOXD1R | GCCTTGAACTCGACCG | 1866-1851 | X77455 | ||
| CMY | CMY25F1 | CAATGTGTGAGAAGCAGTC | 50 | 1,432 | 1736-1754 | X91840 |
| CMY | CMY2DR1 | CGCATGGGATTTTGGTTGCTG | 3167-3147 | X91840 | ||
| ACT-1 | EccKF | CGAACGAATCATTCAGCACCG | 50 | 418 | 421-401 | X04730 |
| ACT-1 | ACT-PE | GCCAATACCGAGCAGGAGGTG | 89-69 | U58495 | ||
| EcAmpR | ENTAmpR1F | CCGTAATAGCGAGTCAAGGG | 50 | 1,066 | 68-87 | X04730 |
| EcAmpR | ENTAmpR1R | CTGACGGTCGTCACGTTGATTGC | 1133-1111 | X04730 |
Target sequence for amplification.
Primer set used for amplification.
Sequence of primer pairs used for amplification.
Annealing temperature for amplification.
Size of amplicon generated for specific primer pair.
Nucleotide (nt) location of primer as it corresponds to the sequence with the indicated GenBank accession number.
RESULTS
Among 1,123 isolates screened at the 24 study sites, 439 (39%) were adjudged to be positive by screening and were referred to Creighton University. Of these, 31 were not confirmed to be K. pneumoniae and were excluded from further testing. The reasons for the misidentifications were not investigated. This left a total of 408 isolates (36.3%) for the β-lactamase production investigations. Of these, 279 (68%) produced at least one of the β-lactamases of interest for this study and 129 (32%) did not. Two hundred thirty-seven (58%) produced an ESBL, 46 (11%) produced an AmpC β-lactamase, 8 (2%) produced an ESBL and an AmpC β-lactamase, and 4 (1%) produced a class A carbapenem-hydrolyzing enzyme (Table 2). All AmpC β-lactamases were considered to be encoded by transmissible genes because the ampC gene is not part of the K. pneumoniae genotype. However, no attempt was made to determine if the ampC genes were located on plasmids, had been inserted into the chromosome, or were transmissible. Therefore, for the purpose of strict scientific accuracy, although the AmpC β-lactamases detected in K. pneumoniae in this study may indeed have been plasmid mediated, they are described by using the less specific adjective “imported” and are referred to as imported AmpC β-lactamases. Not all isolates exhibited β-lactamase-mediated resistance on testing at Creighton University, indicating that some of the positive screening results were due to other resistance mechanisms, such as reduced outer membrane permeability, or that some isolates may have spontaneously lost β-lactamase-encoding plasmids.
TABLE 2.
Numbers and percentages of β-lactamase types detected among 408 isolates obtained from 24 study sites
| Isolates that express β-lactamases | No. of sites with isolates/total no. of sites (%) | No. of isolates producing β-lactamase/total no. of isolates (%) |
|---|---|---|
| ESBL-producing isolates | 18/24 (75) | 237/408 (58) |
| Imported AmpC-producing isolates | 10/24 (42) | 46/408 (11) |
| Inducible imported AmpC-producing isolates | 3/24 (12.5) | 4/408 (1) |
| ESBL-producing and imported AmpC-producing isolates | 9/24 (38) | |
| Isolates coproducing ESBLs and imported AmpC β-lactamases | 8/408 (2) | |
| Class A carbapeneming-hydrolyzing isolates | 1/24 (4) | 4/408 (1) |
| Class B or D carbapenem-hydrolyzing isolates | 0 (0) | 0 (0) |
Susceptibility data for the 408 isolates tested at Creighton University are summarized in Table 3. The carbapenems ertapenem and imipenem (MICs at which 90% of isolates are inhibited [MIC90s], 0.25 and 1 μg/ml, respectively), were the most potent agents overall. All other agents had off-scale MIC90s, but on the basis of the MIC50s, the next most potent agents were (MIC50s are given in parentheses) cefepime (2 μg/ml), cefoxitin (8 μg/ml), piperacillin-tazobactam (≤16/4 μg/ml), and ceftriaxone and cefotaxime (both 16 μg/ml). The MIC50s of aztreonam and ceftazidime were off scale (>16 and >64 μg/ml, respectively).
TABLE 3.
Susceptibility data
| Organism (no.) and agent | MIC (μg/ml)
|
||
|---|---|---|---|
| Range | 50% | 90% | |
| All isolates (408) | |||
| Ertapenem | ≤0.008->8 | 0.06 | 0.25 |
| Imipenem | ≤0.06-8 | 0.5 | 1 |
| Ceftriaxone | ≤0.06->64 | 16 | >64 |
| Ceftazidime | ≤0.5->64 | >64 | >64 |
| Cefotaxime | ≤0.5->32 | 16 | >32 |
| Aztreonam | ≤0.5->16 | >16 | >16 |
| Cefoxitin | ≤0.5->16 | 8 | >16 |
| Cefepime | ≤0.25->16 | 2 | >16 |
| Piperacillin-tazobactam | ≤16->64 | ≤16 | >64 |
| ESBL-producing isolates (237) | |||
| Ertapenem | ≤0.008->8 | 0.06 | 0.25 |
| Imipenem | ≤0.06-2 | 0.25 | 1 |
| Ceftriaxone | 0.12->64 | >64 | >64 |
| Ceftazidime | ≤0.05->64 | >64 | >64 |
| Cefotaxime | ≤0.5->32 | >32 | >32 |
| Aztreonam | ≤0.5->16 | >16 | >16 |
| Cefoxitin | ≤0.5->16 | 8 | >16 |
| Cefepime | ≤0.25->16 | >16 | >16 |
| Piperacillin-tazobactam | ≤16->64 | 32 | >64 |
| Imported AmpC-producing isolates (46) | |||
| Ertapenem | 0.015-1 | 0.06 | 0.5 |
| Imipenem | 0.12-2 | 0.5 | 1 |
| Ceftriaxone | 2->64 | 16 | 64 |
| Ceftazidime | 8->64 | 32 | >64 |
| Cefotaxime | ≤0.5->32 | 8 | >32 |
| Aztreonam | ≤0.5->16 | 4 | >16 |
| Cefoxitin | ≤0.5->16 | >16 | >16 |
| Cefepime | ≤0.25->16 | 1 | 4 |
| Piperacillin-tazobactam | ≤16->64 | ≤16 | >64 |
| KPC-2-producing isolates (4) | |||
| Ertapenem | 8->8 | ||
| Imipenem | 2-8 | ||
| Ceftriaxone | 64->64 | ||
| Ceftazidime | 4->64 | ||
| Cefotaxime | 16->32 | ||
| Aztreonam | >16 | ||
| Cefoxitin | 4->16 | ||
| Cefepime | 4->16 | ||
| Piperacillin-tazobactam | 32->64 | ||
ESBL production.
ESBL production was confirmed in 237 of the 408 isolates (58%). The isolates were from 18 of the 24 sites (75%) (Table 2). The rates of ESBL production varied among the sites, ranging from 0 to 73%. Ertapenem and imipenem were the only agents with significant activities against these isolates, with MIC90s of 0.25 and 1 μg/ml, respectively (Table 3). Cefoxitin and piperacillin-tazobactam were the next most active agents, with MIC50s of 8 and 32 μg/ml, respectively (MIC90s were off-scale), while the cephalosporins and aztreonam were the least active agents, with off-scale MIC50s and MIC90s.
Imported AmpC β-lactamases.
Cefoxitin MICs were ≥16 μg/ml for 160 of the 408 isolates, and the isolates were investigated for AmpC production. Of these, AmpC β-lactamase production was confirmed in 46 isolates, i.e., 11% of the 408 isolates or 28.8% of the 160 cefoxitin screening-positive isolates. The MICs for significant numbers of AmpC-producing isolates indicated that they were susceptible to ceftriaxone (59%), cefotaxime (50%), and aztreonam (70%); but only 1 of the 46 isolates (2.1%) was susceptible to ceftazidime (7 isolates were intermediate). Thus, a pattern emerged in which cefoxitin and ceftazidime MICs ≥16 μg/ml were observed for 45 of 46 (98%) AmpC-producing isolates. Unfortunately, from a diagnostic viewpoint, this simple phenotype was not specific, as it also occurred with 36 non-AmpC-producing isolates (33 ESBL producers; 1 carbapenem-hydrolyzing enzyme producer; and 2 isolates that did not produce an AmpC, ESBL, or carbapenem-hydrolyzing enzyme). However, if these cefoxitin and ceftazidime MIC criteria were combined with the additional criterion of a negative confirmatory test result for ESBL production, 45 of 46 (97.8%) of the AmpC-producing K. pneumoniae isolates were identified, with only an additional 3 AmpC-negative isolates meeting the criteria. That is, 45 of 48 isolates (93.8%) that met the criteria of cefoxitin and ceftazidime MICs of ≥16 μg/ml and negative confirmatory test results for ESBL production were positive for AmpC production.
The AmpC-producing isolates were obtained from 10 of the 24 sites (42%) (Table 2). Eighty-nine percent of the 46 isolates were from cities in eastern states: New York, N.Y. (n = 12 isolates); Louisville, Ky. (n = 10); Memphis, Tenn. (n = 10); Miami, Fla. (n = 6); Baltimore, Md. (n = 2); and Cleveland, Ohio (n = 1). Only five isolates (11%) were from West Coast cities: Portland, Oreg. (n = 4), and Seattle, Wash. (n = 1). These findings probably only reflect the fact that only 3 of the 24 sites in the study were on the West Coast (Fig. 1). Nine sites (38%) had both ESBL-producing and AmpC-producing isolates: Baltimore, Md.; Miami, Fla.; Louisville, Ky.; New York, N.Y. (three sites); Cleveland, Ohio; Portland, Oreg.; and Memphis, Tenn. Eight isolates from Louisville, Ky. (n = 3), New York, N.Y. (n = 1), and Memphis, Tenn. (n = 4), produced both an ESBL and an imported AmpC β-lactamase. Each of the isolates that coproduced an ESBL and an AmpC β-lactamase yielded a positive confirmatory test result for ESBL production with the ceftriaxone and ceftriaxone-clavulanate approach, but only one of the eight isolates yielded a positive confirmatory test result with the ceftazidime and ceftazidime-clavulanate approach.
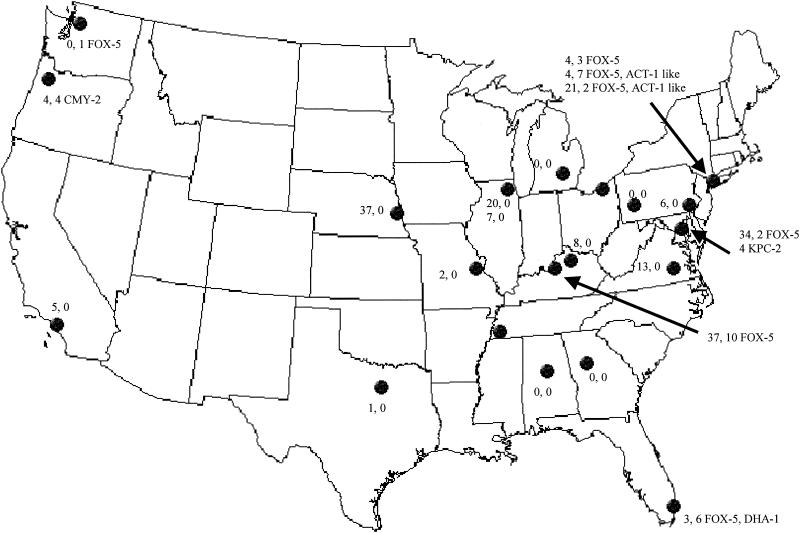
FIG. 1.
Map showing sites included in the study and the numbers of ESBL-producing, AmpC-producing, and carbapenem-hydrolyzing, enzyme-producing isolates at each site and the types of AmpC and carbapenem-hydrolyzing β-lactamases detected. The first number indicates the number of ESBL-positive isolates; the second number and designation indicate the number of AmpC-positive isolates and the type(s) of AmpC, and the third number indicates the number of isolates producing the carbapenem-hydrolyzing enzyme KPC-2.
Ertapenem, imipenem, and cefepime were the most active agents against the imported AmpC-producing strains, with MIC90s of 0.5, 1, and 4 μg/ml, respectively (Table 3). Aztreonam, cefotaxime, and piperacillin-tazobactam were the next most active agents, with MIC50s of 4, 8, and ≤16 μg/ml, respectively (MIC90s were off-scale).
By IEF the AmpC β-lactamases exhibited three pI values: 7.2, 7.7, and >9.0. Isolates from 3 of the 10 sites had two types of imported AmpC β-lactamases, with isolates at each of these sites having an inducible AmpC enzyme. The inducible AmpC enzymes had pI values of 7.7 (one isolate from Miami, Fla.) and >9.0 (three isolates from two New York City hospitals). The inducible enzyme with a pI of 7.7 was confirmed to be DHA-1 by a blaDHA-1-specific PCR and sequencing, and the inducible enzymes with pIs of >9.0 were confirmed to be ACT-1-like by a blaACT-1-specific PCR. In addition, DNA templates from these isolates also amplified an E. cloacae ampR-specific PCR amplicon. Another enzyme with a pI of >9.0 (not inducible) from a Portland, Oreg., isolate was confirmed to be CMY-2 by a blaCMY-specific PCR and sequencing. The AmpC β-lactamases that had pIs of 7.2 were identified as FOX-5 by PCR amplification and sequencing.
Carbapenem-hydrolyzing enzymes.
The imipenem MIC was ≥1 μg/ml for 72 isolates, and the isolates were evaluated further for production of carbapenem-hydrolyzing enzymes. Of these 72 isolates, the 63 isolates (87.5%) for which the imipenem MIC was 1 μg/ml did not produce a carbapenem-hydrolyzing enzyme, suggesting that the screening criterion of an imipenem MIC of ≥1 μg/ml was too sensitive. One site submitted four unrelated isolates that produced the KPC-2 molecular class A carbapenem-hydrolyzing enzyme (E. S. Moland, J. A. Black, J. Johnson, T. J. Lockhart, A Hossian, V. L. Herrera, N. D. Hanson, and K. S. Thomson, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2226, 2001). A screening criterion of an imipenem MIC of ≥2 μg/ml would have detected all of these isolates and yielded an additional five isolates that met this criterion. All KPC-2-producing isolates produced multiple β-lactamases. Imipenem was the most potent agent overall against these strains, with MICs in the range of 2 to 8 μg/ml (Table 3). The range of MICs of the other antibiotics varied widely, possibly reflecting differences between the isolates in the types of β-lactamases that they produced, their levels of KPC-2 expression, and other resistance mechanisms. No class B enzymes (metallo-β-lactamases) or class D carbapenem-hydrolyzing enzymes were detected.
DISCUSSION
It is critical that clinical microbiology laboratories be able to detect ESBLs, imported AmpC, and carbapenem-hydrolyzing β-lactamases because these enzymes are either associated with problems of false susceptibility or have significant infection control implications, or both. In this study the occurrence of isolates that produce ESBLs at 75% of the sites, imported AmpC β-lactamases at 42% of the sites, and both types of enzymes at 38% of the sites is indicative of the significant penetration of these two types of β-lactamases into K. pneumoniae in U.S. medical institutions. As this was an occurrence study, not a prevalence study, the data accurately indicate only the presence or the absence of β-lactamase types. That is, particular enzymes were either detected or not detected in the K. pneumoniae isolates at the study sites. The data do not indicate the prevalence of a particular enzyme type at any site or the prevalence across the United States. However, since the study was designed to enhance the likelihood of detection of imported AmpC β-lactamases and carbapenem-hydrolyzing enzymes by giving laboratories the option of including archived isolates of K. pneumoniae (1996 to 2000) that were intermediate or resistant to either cefoxitin or imipenem, the confirmation that only 46 isolates were imported AmpC producers (whereas 237 isolates were ESBL producers) suggests that while isolates producing imported AmpC β-lactamases are fairly widespread, they appear to be less prevalent than isolates producing ESBLs.
Three sites had isolates that produced inducible imported AmpC β-lactamases. There are only three previous reports of these types of enzymes. In 1998 French investigators reported that DHA-1 was inducible in a single isolate of Salmonella enterica subsp. enterica serovar Enteritidis (2). A related inducible enzyme, DHA-2, was reported in a single isolate of K. pneumoniae in 2001, again by French investigators (12), and ACT-1 was reported as inducible in an isolate of K. pneumoniae from New York, N.Y., in 2002 (27). In this study the fact that an additional four isolates produced inducible imported AmpC β-lactamases should increase awareness of this type of enzyme. Three isolates from New York, N.Y., produced an inducible ACT-1-like enzyme, and one isolate from Miami, Fla., produced an inducible DHA-1 enzyme. The clinical implications of these types of enzymes are unknown. Since inducible expression of chromosomal AmpC β-lactamases is associated with a significant risk of therapeutic failure with all β-lactam drugs except carbapenems and dipolar methoxyimino-cephalosporins (28, 32), the question arises whether inducible expression of plasmid-mediated AmpC β-lactamases confers a similar or greater risk of therapeutic failure than constitutive expression of plasmid-mediated AmpC β-lactamases. If the risk is greater, it will be important for clinical laboratories not only to be able to detect imported AmpC β-lactamases but also to be able to test for inducibility. This will be especially important if the resistance caused by the inducible enzymes is not reliably detected in routine susceptibility tests.
From the diagnostic standpoint, it was interesting that isolates for which cefoxitin and ceftazidime MICs were ≥16 μg/ml and which had negative confirmatory test results for ESBL production comprised a subgroup of 48 isolates, of which 45 were confirmed to be producers of an AmpC β-lactamase. Only one AmpC-producing isolate was not detected by use of these criteria (the ceftazidime MIC for the isolate was lower [8 μg/ml]). Further investigation of this phenotypic screening method for AmpC production seems warranted, as it offers the potential to significantly reduce the number of isolates that would require a confirmatory test for AmpC production, such as the three-dimensional test with cefoxitin (35) or a test involving a β-lactamase inhibitor specific for AmpC β-lactamases (10).
Although carbapenem-hydrolyzing enzymes were sought among archived isolates, their occurrence was rare. No metallo-β-lactamases or class D carbapenem-hydrolyzing enzymes were detected. The class A carbapenem-hydrolyzing enzyme KPC-2 was detected in four isolates from one site. The significance of this enzyme for carbapenem therapy is unknown. In the four isolates, this enzyme was associated with elevated carbapenem MICs but not imipenem resistance (Moland et al., 41st ICAAC). Therefore to detect this enzyme, it is important for clinical laboratories to be vigilant for isolates with decreased susceptibilities to carbapenems, in this case, an imipenem MIC of ≥2 μg/ml.
The finding that the carbapenems ertapenem and imipenem were the most potent agents overall (MIC90s, 0.25 and 1 μg/ml, respectively) against isolates chosen for their reduced susceptibilities to β-lactam antibiotics was not unexpected. In this, our data confirmed the findings of two previous studies, one of 181 European isolates of ESBL-producing Klebsiella spp. (17) and the other of 76 oxyimino-β-lactam-resistant isolates of the family Enterobacteriaceae that either produced ESBLs or overproduced a chromosomal AmpC β-lactamase from 32 U.S. hospitals (14).
In conclusion, this study demonstrated the widespread occurrence of ESBLs and imported AmpC β-lactamases and the rare occurrence of a class A carbapenem-hydrolyzing enzyme in K. pneumoniae isolates in U.S. medical institutions. Because novel β-lactamases may significantly affect therapeutic outcomes, it is critical that clinical laboratories be able to detect their occurrence. It is important that laboratory personnel and physicians continue to be made aware of the therapeutic and infection control implications of not detecting ESBLs, imported AmpC β-lactamases, and other novel β-lactamases such as carbapenem-hydrolyzing enzymes.
Acknowledgments
This study was supported in part by a grant from Merck & Co., Inc.
We thank T. J. Lockhart for excellent technical assistance, V. Herrera for providing molecular testing of the isolates that produced KPC-2, and the investigators comprising the Newer β-Lactamases Study Group and their institutions. They are (in alphabetical order by state) K. Waites, University of Alabama, Birmingham; J. Hindler, University of California at Los Angeles; T. Cleary, Jackson Memorial Hospital, Miami, Fla.; F. Nolte, Emory University, Atlanta, Ga.; R. Carey, Loyola University, Chicago, Ill.; P. Verma, Rush Presbyterian St. Lukes Hospital, Chicago, Ill.; S. Overman, University of Kentucky Hospital, Lexington; J. Snyder, University of Louisville Hospital, Louisville, Ky.; J. Johnson, Baltimore Veterans Administration Medical Center, Baltimore, Md.; C. Pierson, University of Michigan Hospitals, Ann Arbor, Mich.; J. Hoppe-Bauer, Barnes/Jewish Hospital, St. Louis, Mo.; P. Fey, University of Nebraska Medical Center, Omaha; M. Pincus, Harbor Veterans Administration Medical Center, Brooklyn, N.Y.; B. Hanna, New York University Bellevue Hospital, New York, N.Y.; C. Ginocchio, North Shore-Long Island Jewish Health Care System, Lake Success, N.Y.; G. Hall, Cleveland Clinic Foundation, Cleveland, Ohio; D. Sewell, Portland Veterans Administration Medical Center, Portland, Oreg.; J. Rihs, Pittsburgh Veterans Administration Medical Center, Pittsburgh, Pa.; B. Suh, Temple University Hospital, Philadelphia, Pa.; S. Davis, Methodist Healthcare, Memphis, Tenn.; P. Southern, University of Texas Southwestern Medical Center, Dallas; P. Coudron, McGuire Veterans Administration Medical Center, Richmond, Va.; and T. Fritsche, University of Washington, Seattle.
REFERENCES
- 1.Afzal-Shah, M., H. E. Villar, and D. M. Livermore. 1999. Biochemical characteristics of a carbapenemase from an Acinetobacter baumannii isolate collected in Buenos Aires, Argentina. J. Antimicrob. Chemother. 43:127-131. [DOI] [PubMed] [Google Scholar]
- 2.Barnaud, G., G. Arlet, C. Verdet, O. Gaillot, P. H. Lagrange, and A. Philippon. 1998. Salmonella enteritidis: AmpC plasmid-mediated inducible β-lactamase (DHA-1) with an ampR gene from Morganella morganii. Antimicrob. Agents Chemother. 42:2352-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauernfeind, A., Y. Chong, and K. Lee. 1998. Plasmid-encoded AmpC beta-lactamases: how far have we gone 10 years after the discovery? Yonsei Med. J. 39:520-525. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind, A., Y. Chong, and S. Schweighart. 1989. Extended broad-spectrum β-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection 17:316-321. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, P. A. 2001. What's new in β-lactamases? Curr. Infect. Dis. Rep. 3:13-19. [DOI] [PubMed] [Google Scholar]
- 6.Brun-Buisson, C., P. Legrand, A. Philippon, F. Montravers, M. Ansquer, and J. Duval. 1987. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreak of multiresistant Klebsiella pneumoniae. Lancet ii:302-306. [DOI] [PubMed]
- 7.Bush, K. 1998. Metallo-beta-lactamases: a class apart. Clin. Infect. Dis. 27(Suppl. 1):S48-S53. [DOI] [PubMed] [Google Scholar]
- 8.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casellas, J. M., and M. Goldberg. 1989. Incidence of strains producing extended spectrum β-lactamases in Argentina. Infection 17:434-436. [DOI] [PubMed] [Google Scholar]
- 10.Coudron, P. E., E. S. Moland, and K. S. Thomson. 2000. Occurrence and detection of AmpC β-lactamases among Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates at a veterans medical center. J. Clin. Microbiol. 38:1791-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felici, A., and G. Amicosante. 1995. Kinetic analysis of extension of substrate specificity with Xanthomonas maltophilia, Aeromonas hydrophila, and Bacillus cereus metallo-β-lactamases. Antimicrob. Agents Chemother. 39:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortineau, N., L. Poirel, and P. Nordmann. 2001. Plasmid-mediated and inducible cephalosporinase DHA-2 from Klebsiella pneumoniae. J. Antimicrob. Chemother. 47:207-210. [DOI] [PubMed] [Google Scholar]
- 13.Hanson, N. D., E. S. Moland, A. Hossain, S. A. Neville, I. B. Gosbell, and K. S. Thomson. 2002. Unusual Salmonella enterica serotype Typhimurium isolate producing CMY-7, SHV-9 and OXA-30 beta-lactamases. J. Antimicrob. Chemother. 49:1011-1014. [DOI] [PubMed] [Google Scholar]
- 14.Jacoby, G., P. Han, and J. Tran. 1997. Comparative in vitro activities of carbapenem L-749,345 and other antimicrobials against multiresistant gram-negative clinical pathogens. Antimicrob. Agents Chemother. 41:1830-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacoby, G. A., and A. A. Medeiros. 1991. More extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 35:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karas, J. A., D. G. Pillay, D. Muckart, and A. W. Sturm. 1996. Treatment failure due to extended spectrum β-lactamase. J. Antimicrob. Chemother. 37:203-204. [DOI] [PubMed] [Google Scholar]
- 17.Livermore, D. M., K. J. Oakton, M. W. Carter, and M. Warner. 2001. Activity of ertapenem (MK-0826) versus Enterobacteriaceae with potent β-lactamases. Antimicrob. Agents Chemother. 45:2831-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livermore, D. M., and N. Woodford. 2000. Carbapenemases: a problem in waiting? Curr. Opin. Microbiol. 3:489-495. [DOI] [PubMed] [Google Scholar]
- 19.Masuda, G., S. Tomioka, and M. Hasegawa. 1976. Detection of beta-lactamase production by gram-negative bacteria. J. Antibiot. (Tokyo) 29:662-664. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing; 10th informational supplement (aerobic dilution) M100-S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 21.Nordmann, P., and M. Guibert. 1998. Extended-spectrum beta-lactamases in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 42:128-131. [DOI] [PubMed] [Google Scholar]
- 22.Paterson, D. L., N. Singh, T. Gayowski, and I. R. Marino. 1999. Fatal infection due to extended-spectrum beta-lactamase-producing Escherichia coli: implications for antibiotic choice for spontaneous bacterial peritonitis. Clin. Infect. Dis. 28:683-684. [DOI] [PubMed] [Google Scholar]
- 23.Philippon, A., G. Arlet, and G. A. Jacoby. 2002. Plasmid-determined AmpC-type β-lactamases. Antimicrob. Agents Chemother. 46:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philippon, A., R. Labia, and G. A. Jacoby. 1989. Extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 33:1131-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahal, J. J. 2000. Extended-spectrum beta-lactamases: how big is the problem? Clin. Microbiol. Infect. 2(Suppl. 2):2-6. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen, B. A., and K. Bush. 1997. Carbapenem-hydrolyzing β-lactamases. Antimicrob. Agents Chemother. 41:223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reisbig, M. D., and N. D. Hanson. 2002. The ACT-1 plasmid-encoded AmpC beta-lactamase is inducible: detection in a complex beta-lactamase background. J. Antimicrob. Chemother. 49:557-560. [DOI] [PubMed] [Google Scholar]
- 28.Sanders, C. C., and W. E. Sanders, Jr. 1992. β-Lactam resistance in gram-negative bacteria: global trends and clinical impact. Clin. Infect. Dis. 15:824-839. [DOI] [PubMed] [Google Scholar]
- 29.Sanders, C. C., W. E. Sanders, Jr., and E. S. Moland. 1986. Characterization of β-lactamases in situ on polyacrylamide gels. Antimicrob. Agents Chemother. 30:951-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders, C. C., and W. E. Sanders, Jr. 1979. Emergence of resistance to cefamandole: possible role of cefoxitin-inducible β-lactamases. Antimicrob. Agents Chemother. 15:792-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders, C. C., W. E. Sanders, Jr., K. S. Thomson, and J. P. Iaconis. 1989. Meropenem: activity against resistant gram-negative bacteria and interactions with beta-lactamases. J. Antimicrob. Chemother. 24(Suppl. A):187-196. [DOI] [PubMed] [Google Scholar]
- 32.Sanders, W. E., J. H. Tenney, and R. E. Kessler. 1996. Efficacy of cefepime in the treatment of infections due to multiply resistant Enterobacter spp. Clin. Infect. Dis. 23:454-461. [DOI] [PubMed] [Google Scholar]
- 33.Sirot, D. 1995. Extended-spectrum plasmid-mediated β-lactamases. J. Antimicrob. Chemother. 36:19-34. [DOI] [PubMed] [Google Scholar]
- 34.Tenover, F. C., M. J. Mohammed, T. S. Gorton, and Z. F. Dembek. 1999. Detection and reporting of organisms producing extended-spectrum β-lactamases: survey of laboratories in Connecticut. J. Clin. Microbiol. 37:4065-4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson, K. S., and C. C. Sanders. 1992. Detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae: comparison of the double-disk and three-dimensional tests. Antimicrob. Agents Chemother. 36:1877-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson, K. S., C. C. Sanders, and E. S. Moland. 1999. Use of microdilution panels with and without β-lactamase inhibitors as a phenotypic test for β-lactamase production among Escherichia coli, Klebsiella spp., Enterobacter spp., Citrobacter freundii, and Serratia marcescens. Antimicrob. Agents Chemother. 43:1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomson, K. S., and E. Smith Moland. 2000. Version 2000: the new beta-lactamases of gram-negative bacteria at the dawn of the new millennium. Microbes Infect. 2:1225-1235. [DOI] [PubMed] [Google Scholar]
- 38.Venezia, R. A., F. J. Scarano, K. E. Preston, L. M. Steele, T. P. Root, R. Limberger, W. Archinal, and M. A. Kacica. 1995. Molecular epidemiology of an SHV-5 extended-spectrum beta-lactamase in Enterobacteriaceae isolated from infants in a neonatal intensive care unit. Clin. Infect. Dis. 21:915-923. [DOI] [PubMed] [Google Scholar]