Abstract
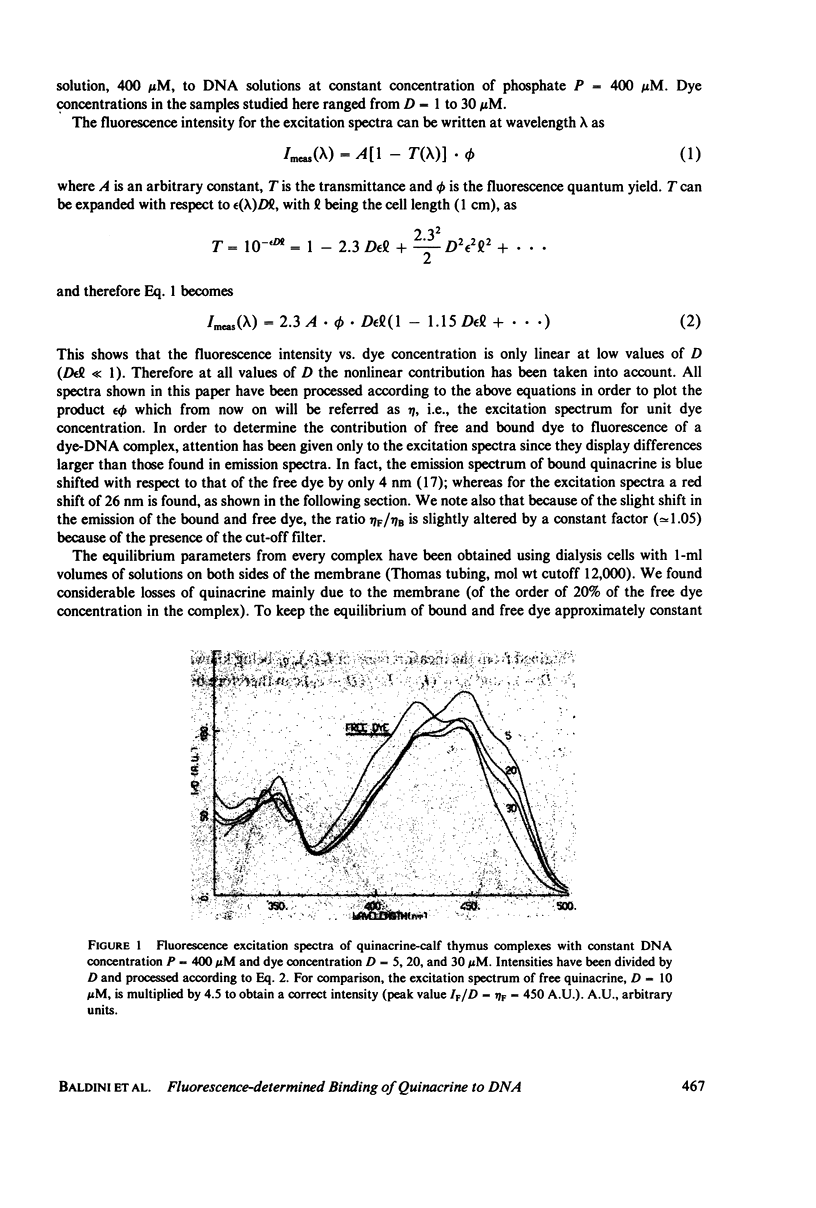
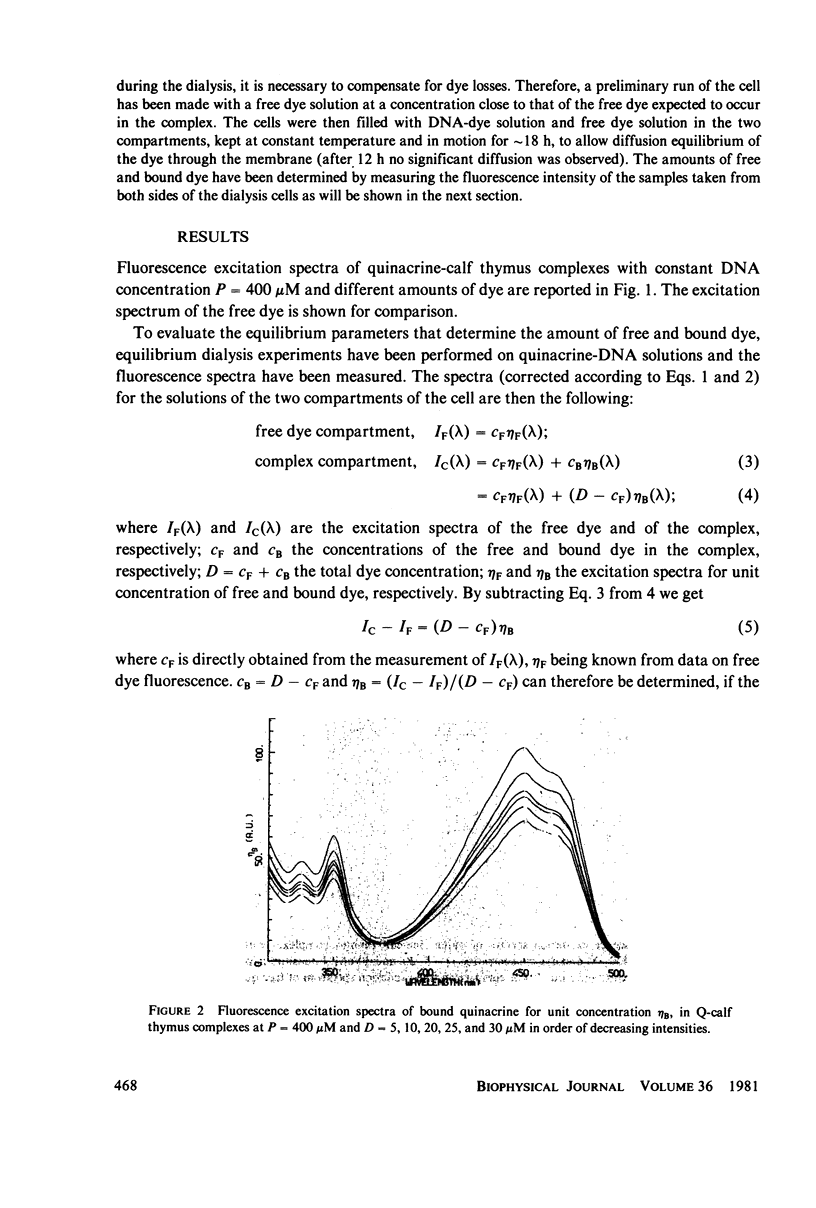
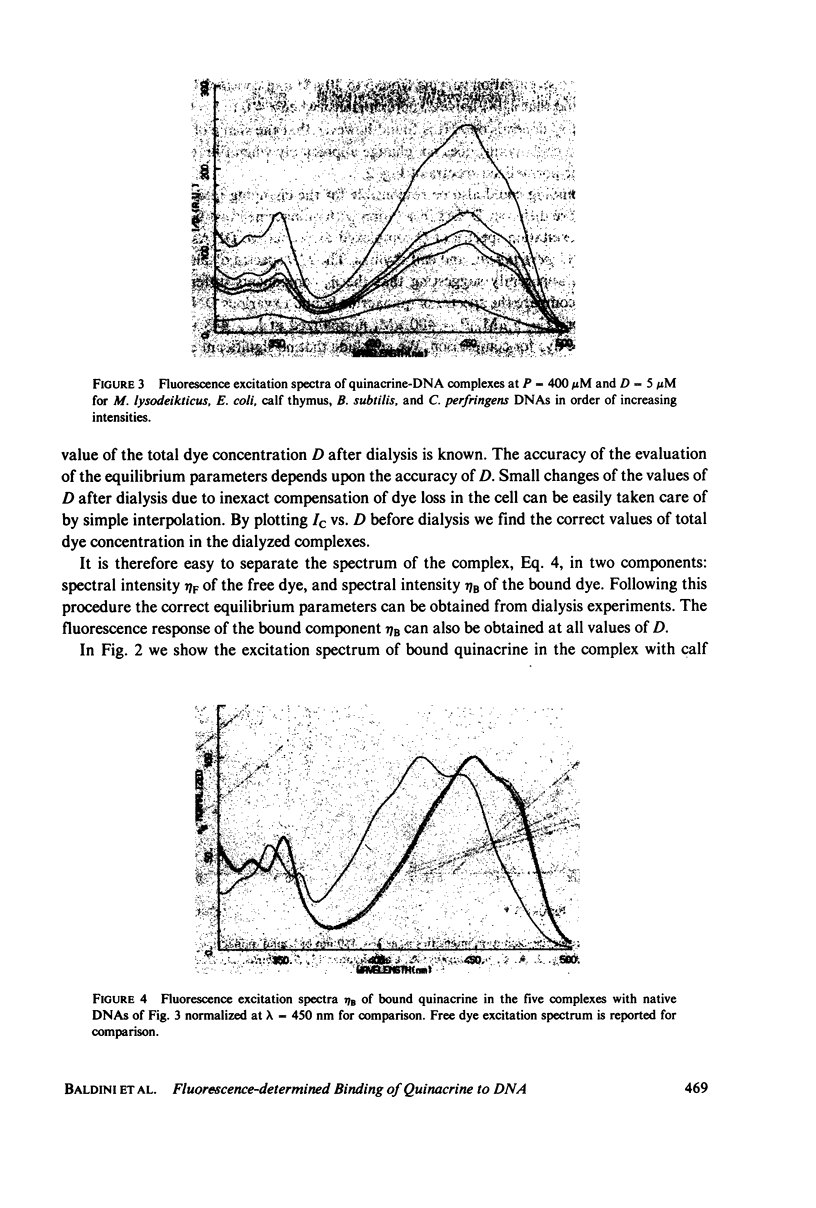
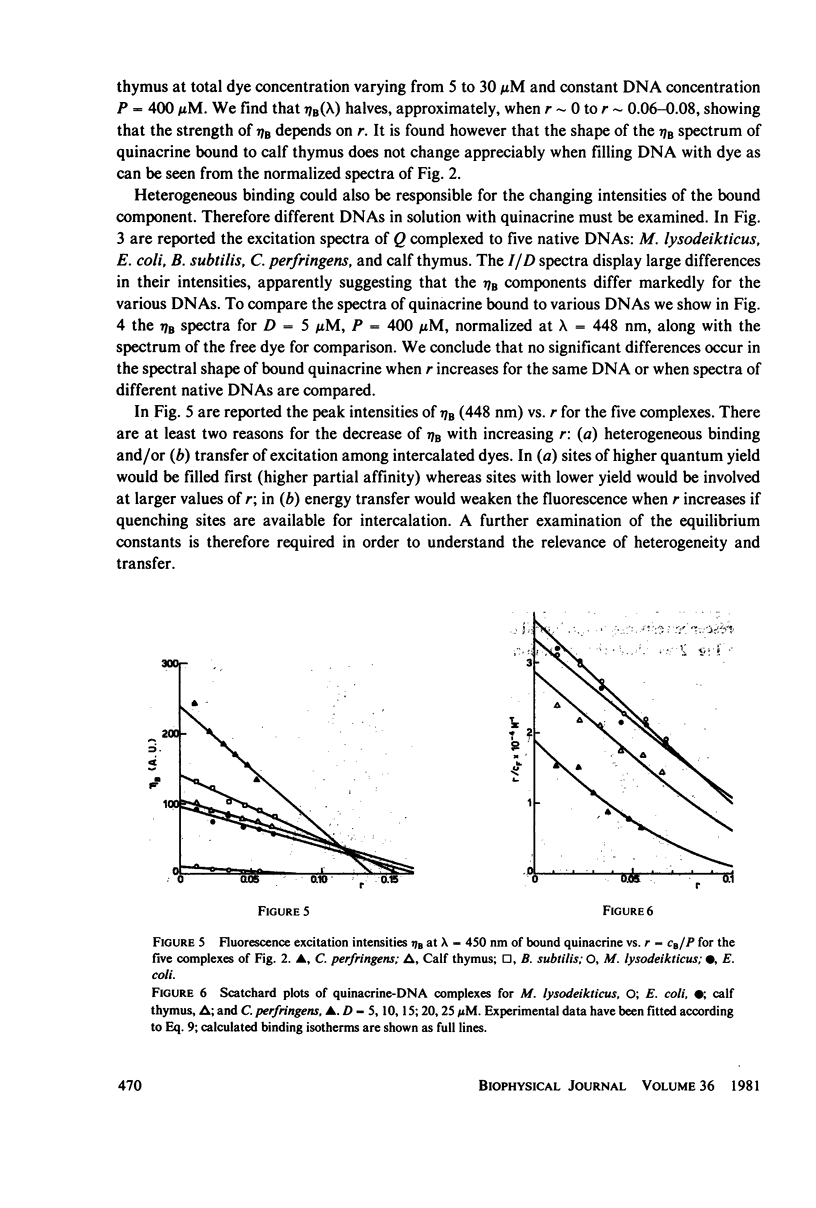
Quinacrine complexes with native DNA (Calf thymus, Micrococcus lysodeikticus, Escherichia coli, Bacillus subtilis, and Colstridium perfringens) and synthetic polynucleotides (poly(dA) . poly(dT), poly[d(A-T)] . poly[d(A-T)], poly(dG) . poly(dC) and poly[d(G-C)] . poly[d(G-C)]) has been investigated in solution at 0.1 M NaCl, 0.05 M Tris HCl, 0.001 M EDTA, pH 7.5, at 20 degrees C. Fluorescence excitation spectra of complexes with dye concentration D = 5-30 microM and DNA phosphate concentration P = 400 microM have been examined from 300 to 500 nm, while collecting the emission above 520 nm. The amounts of free and bound quinacrine in the dye-DNA complexes have been determined by means of equilibrium dialysis experiments. Different affinities have been found for the various DNAs and their values have been examined with a model that assumes that the binding constants associated with alternating purine and pyrimidine sequences are larger than those relative to nonalternating ones. Among the alternating nearest neighbor base sequences, the Pyr(3'-5')Pur sequences, i.e., C-G, T-G, C-A and T-A seem to bind quinacrine stronger than the remaining sequences. In particular the three sites, where a G . C base pair is involved, are found to display higher affinities. Good agreement is found with recent calculations on the energetics of intercalation sites in DNA. The analysis of the equilibrium shows also that the strength of the excitation spectrum of bound dye depends strongly upon the ratio of bound quinacrine to DNA. This effect can be attributed to dye-dye energy transfer along DNA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Latt S. A., Striker G., Jovin T. M. Fluorescence decay analysis in solution and in a microscope of DNA and chromosomes stained with quinacrine. J Histochem Cytochem. 1979 Jan;27(1):87–95. doi: 10.1177/27.1.438507. [DOI] [PubMed] [Google Scholar]
- Arnott S., Chandrasekaran R., Hukins D. W., Smith P. J., Watts L. Structural details of double-helix observed for DNAs containing alternating purine and pyrimidine sequences. J Mol Biol. 1974 Sep 15;88(2):523–533. doi: 10.1016/0022-2836(74)90499-9. [DOI] [PubMed] [Google Scholar]
- Borisova O. F., Razjivin A. P., Zaregorodzev V. I. Evidence for the quinacrine fluorescence on three AT pairs of DNA. FEBS Lett. 1974 Sep 15;46(1):239–242. doi: 10.1016/0014-5793(74)80377-7. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Farber S., Foley G. E., Kudynowski J., Modest E. J., Simonsson E., Wagh U., Zech L. Chemical differentiation along metaphase chromosomes. Exp Cell Res. 1968 Jan;49(1):219–222. doi: 10.1016/0014-4827(68)90538-7. [DOI] [PubMed] [Google Scholar]
- Caspersson T., Lindsten J., Lomakka G., Moller A., Zech L. The use of fluorescence techniques for the recognition of mammalian chromosomes and chromosome regions. Int Rev Exp Pathol. 1972;11:1–72. [PubMed] [Google Scholar]
- Crothers D. M. Calculation of binding isotherms for heterogenous polymers. Biopolymers. 1968 Apr;6(4):575–584. doi: 10.1002/bip.1968.360060411. [DOI] [PubMed] [Google Scholar]
- Duportail G., Mauss Y., Chambron J. Quantum yields and fluorescence lifetimes of acridine derivatives interacting with DNA. Biopolymers. 1977 Jul;16(7):1397–1413. doi: 10.1002/bip.1977.360160703. [DOI] [PubMed] [Google Scholar]
- Ellison J. R., Barr H. J. Quinacrine fluorescence of specific chromosome regions. Late replication and high A: T content in Samoaia leonensis. Chromosoma. 1972;36(4):375–390. doi: 10.1007/BF00336794. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G., Hirschman S. Z. A neighbor-interaction analysis of the hypochromism and spectra of DNA. J Mol Biol. 1965 Sep;13(2):407–427. doi: 10.1016/s0022-2836(65)80106-1. [DOI] [PubMed] [Google Scholar]
- Giordano P., Bottiroli G., Prenna G., Doglia S., Baldini Low temperature microspectrofluorometry: design of a 'cold chamber'. J Microsc. 1978 Jan;112(1):95–101. doi: 10.1111/j.1365-2818.1978.tb01157.x. [DOI] [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. Helix-random coil transitions in synthetic DNAs of alternating sequence. J Mol Biol. 1962 Aug;5:172–184. doi: 10.1016/s0022-2836(62)80082-5. [DOI] [PubMed] [Google Scholar]
- Krugh T. R., Reinhardt C. G. Evidence for sequence preferences in the intercalative binding of ethidium bromide to dinucleoside monophosphates. J Mol Biol. 1975 Sep 15;97(2):133–162. doi: 10.1016/s0022-2836(75)80031-3. [DOI] [PubMed] [Google Scholar]
- Krugh T. R., Wittlin F. N., Cramer S. P. Ethidium bromide-dinucleotide complexes. Evidence for intercalation and sequence preferences in binding to double-stranded nucleic acids. Biopolymers. 1975 Jan;14(1):197–210. doi: 10.1002/bip.1975.360140114. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Brodie S., Munroe S. H. Optical studies of complexes of quinacrine with DNA and chromatin: implications for the fluorescence of cytological chromosome preparations. Chromosoma. 1974;49(1):17–40. doi: 10.1007/BF00284985. [DOI] [PubMed] [Google Scholar]
- Le Bret M., Le Pecq J. B., Barbet J., Roques B. P. A reexamination of the problem of resonance energy transfer between DNA intercalated chromophores using bisintercalating compounds. Nucleic Acids Res. 1977;4(5):1361–1379. doi: 10.1093/nar/4.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie A. G., Arnott S., Chandrasekaran R., Ratliff R. L. Polymorphism of DNA double helices. J Mol Biol. 1980 Oct 15;143(1):49–72. doi: 10.1016/0022-2836(80)90124-2. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Monny C., Kovoor A. Action of quinacrine mustard on polynucleotides. Biochimie. 1972;54(9):1129–1136. doi: 10.1016/s0300-9084(72)80017-8. [DOI] [PubMed] [Google Scholar]
- Müller W., Bünemann H., Dattagupta N. Interactions of heteroaromatic compounds with nucleic acids. 2. Influence of substituents on the base and sequence specificity of intercalating ligands. Eur J Biochem. 1975 May;54(1):279–291. doi: 10.1111/j.1432-1033.1975.tb04138.x. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Interactions of heteroaromatic compounds with nucleic acids. 1. The influence of heteroatoms and polarizability on the base specificity of intercalating ligands. Eur J Biochem. 1975 May;54(1):267–277. doi: 10.1111/j.1432-1033.1975.tb04137.x. [DOI] [PubMed] [Google Scholar]
- Ornstein R. L., Rein R. Energetics of intercalation specificity. I. Backbone unwinding. Biopolymers. 1979 May;18(5):1277–1291. doi: 10.1002/bip.1979.360180517. [DOI] [PubMed] [Google Scholar]
- Pachmann U., Rigler R. Quantum yield of acridines interacting with DNA of defined sequence. A basis for the explanation of acridine bands in chromosomes. Exp Cell Res. 1972 Jun;72(2):602–608. doi: 10.1016/0014-4827(72)90045-6. [DOI] [PubMed] [Google Scholar]
- Paoletti J., Le Pecq J. B. Resonance energy transfer between ethidium bromide molecules bound to nucleic acids. Does intercalation wind or unwind the DNA helix? J Mol Biol. 1971 Jul 14;59(1):43–62. doi: 10.1016/0022-2836(71)90412-8. [DOI] [PubMed] [Google Scholar]
- TUBBS R. K., DITMARS W. E., Jr, VANWINKLE Q. HETEROGENEITY OF THE INTERACTION OF DNA WITH ACRIFLAVINE. J Mol Biol. 1964 Aug;9:545–557. doi: 10.1016/s0022-2836(64)80226-6. [DOI] [PubMed] [Google Scholar]
- Thomes J. C., Weill G., Daune M. Fluorescence of proflavine--DNA complexes: heterogeneity of binding sites. Biopolymers. 1969;8(5):647–669. doi: 10.1002/bip.1969.360080507. [DOI] [PubMed] [Google Scholar]
- Weisblum B., De Haseth P. L. Quinacrine, a chromosome stain specific for deoxyadenylate-deoxythymidylaterich regions in DNA. Proc Natl Acad Sci U S A. 1972 Mar;69(3):629–632. doi: 10.1073/pnas.69.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Lopp I. G. Analysis of cooperativity and ion effects in the interaction of quinacrine with DNA. Biopolymers. 1979 Dec;18(12):3025–3041. doi: 10.1002/bip.1979.360181210. [DOI] [PubMed] [Google Scholar]


