Abstract
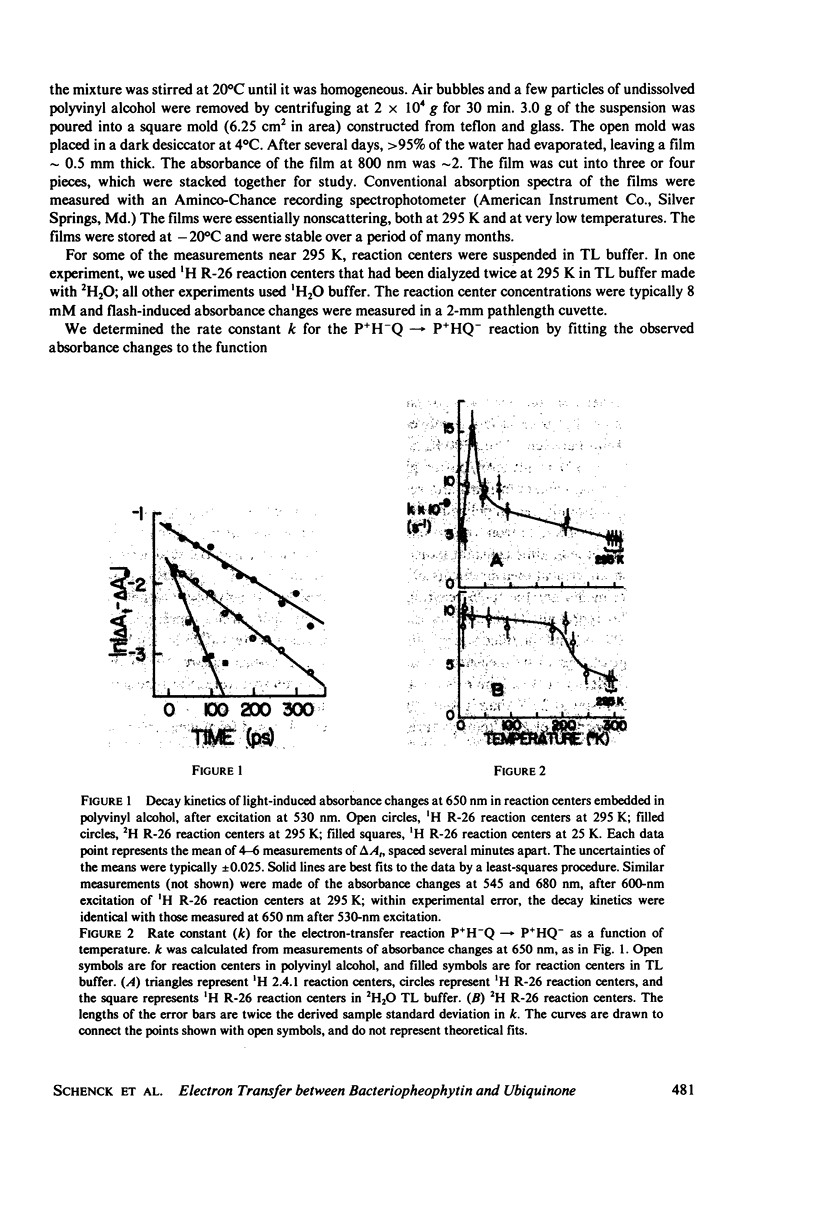
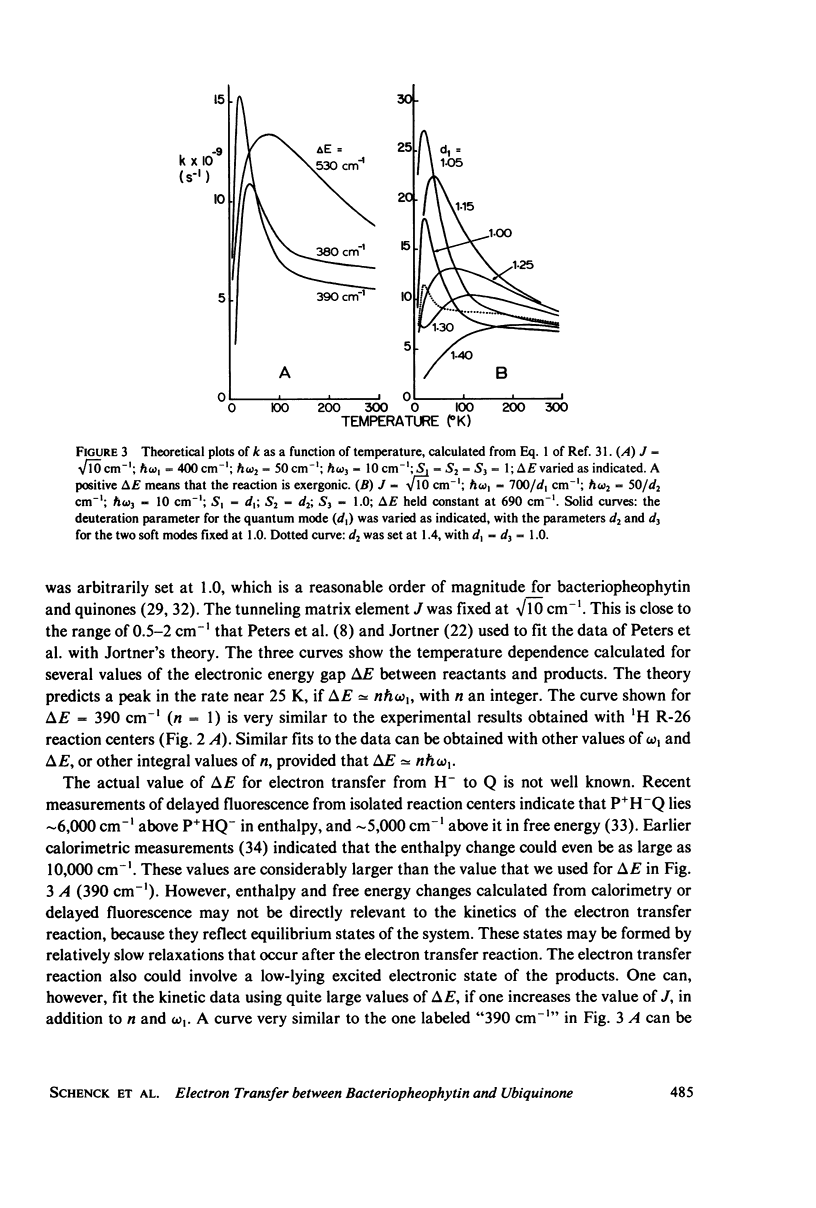
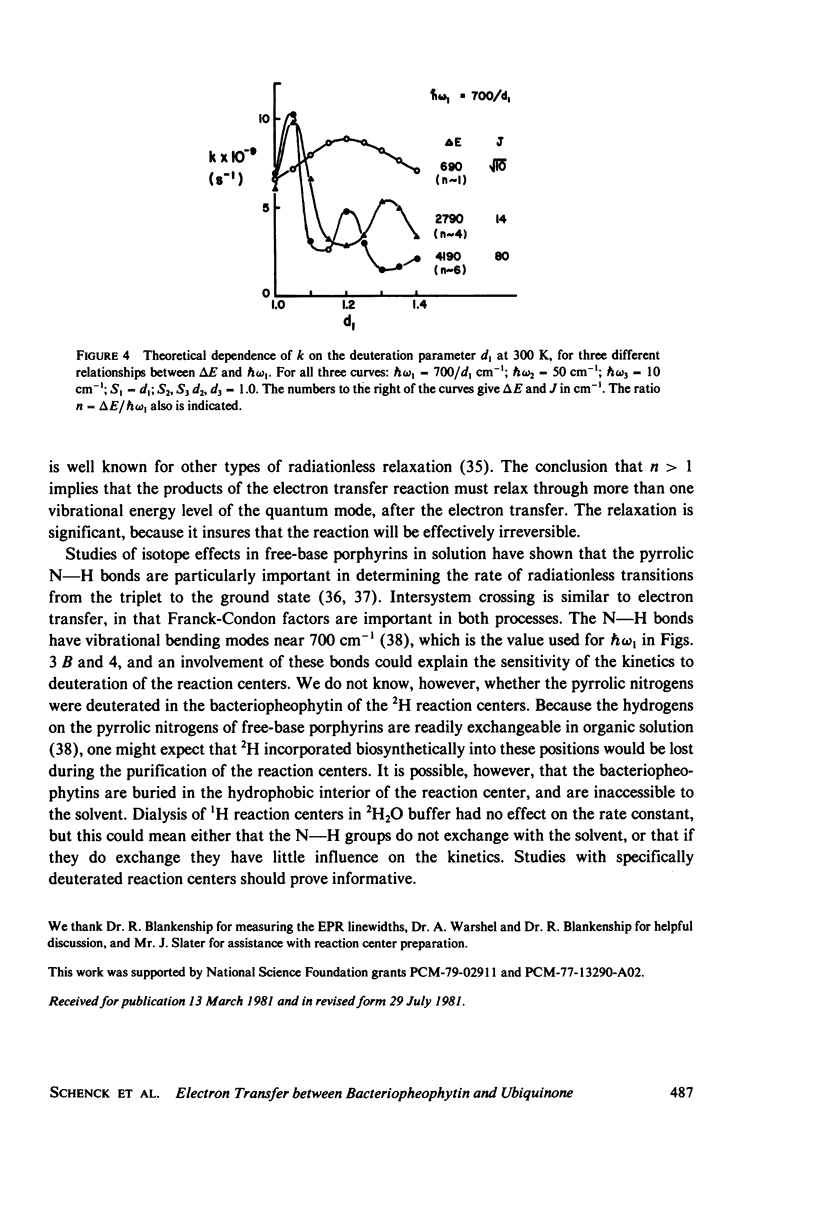
The rate of the electron-transfer reaction between bacteriopheophytin and the first quinone in isolated reaction centers of Rhodopseudomonas sphaeroides has an unusual temperature dependence. The rate increases about threefold with decreasing temperature between 300 and 25 K, and decreases abruptly at temperatures below 25 K. Partial deuteration of the reaction centers alters the temperature dependence of the rate constant. Qualitative features of the temperature dependence can be understood in the context of a theory of nonadiabatic electron transfer (Sarai, 1980. Biochim. Biophys. Acta 589:71-83). We conclude that very low-energy (10-50 cm-1) processes, perhaps skeletal vibrations of the protein, are important to electron transfer. Higher-energy vibrations, possibly involving the pyrrolic N--H bonds of bacteriopheophytin, also are important in this process.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arata H., Parson W. W. Enthalpy and volume changes accompanying electron transfer from P-870 to quinones in Rhodopseudomonas sphaeroides reaction centers. Biochim Biophys Acta. 1981 Jun 12;636(1):70–81. doi: 10.1016/0005-2728(81)90077-3. [DOI] [PubMed] [Google Scholar]
- Blankenship R. E., Parson W. W. The involvement of iron and ubiquinone in electron transfer reactions mediated by reaction centers from photosynthetic bacteria. Biochim Biophys Acta. 1979 Mar 15;545(3):429–444. doi: 10.1016/0005-2728(79)90152-x. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Yau H. F. Photochemical electron transport in photosynthetic reaction centers from Rhodopseudomonas spheroides. I. Kinetics of the oxidation and reduction of P-870 as affected by external factors. Biophys J. 1972 Jul;12(7):867–881. doi: 10.1016/S0006-3495(72)86130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogdell R. J., Monger T. G., Parson W. W. Carotenoid triplet states in reaction centers from Rhodopseudomonas sphaeroides and Rhodospirillum rubrum. Biochim Biophys Acta. 1975 Dec 11;408(3):189–199. doi: 10.1016/0005-2728(75)90122-x. [DOI] [PubMed] [Google Scholar]
- DeVault D., Chance B. Studies of photosynthesis using a pulsed laser. I. Temperature dependence of cytochrome oxidation rate in chromatium. Evidence for tunneling. Biophys J. 1966 Nov;6(6):825–847. doi: 10.1016/s0006-3495(66)86698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault D. Quantum mechanical tunnelling in biological systems. Q Rev Biophys. 1980 Nov;13(4):387–564. doi: 10.1017/s003358350000175x. [DOI] [PubMed] [Google Scholar]
- Holten D., Hoganson C., Windsor M. W., Schenck G. C., Parson W. W., Migus A., Fork R. L., Shank C. V. Subpicosecond and picosecond studies of electron transfer intermediates in Rhodopseudomonas sphaeroides reaction centers. Biochim Biophys Acta. 1980 Oct 3;592(3):461–477. doi: 10.1016/0005-2728(80)90092-4. [DOI] [PubMed] [Google Scholar]
- Holten D., Windsor M. W., Parson W. W., Thornber J. P. Primary photochemical processes in isolated reaction centers of Rhodopseudomonas viridis. Biochim Biophys Acta. 1978 Jan 11;501(1):112–126. doi: 10.1016/0005-2728(78)90100-7. [DOI] [PubMed] [Google Scholar]
- Hsi E. S., Bolton J. R. Flash photolysis-electron spin resonance study of the effect of o-phenanthroline and temperature on the decay time of the ESR signal B1 in reaction-center preparations and chromatophores of mutant and wild strains of Rhodopseudomonas spheroides and Rhodospirillum rubrum. Biochim Biophys Acta. 1974 Apr 23;347(1):126–133. doi: 10.1016/0005-2728(74)90205-9. [DOI] [PubMed] [Google Scholar]
- Kakitani T., Kakitani H. A possible new mechanism of temperature dependence of electron transfer in photosynthetic systems. Biochim Biophys Acta. 1981 May 13;635(3):498–514. doi: 10.1016/0005-2728(81)90109-2. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Dutton P. L., Netzel T. L., Leigh J. S., Rentzepis P. M. Picosecond kinetics of events leading to reaction center bacteriochlorophyll oxidation. Science. 1975 Jun 27;188(4195):1301–1304. doi: 10.1126/science.188.4195.1301. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Peters K., Avouris P., Rentzepis P. M. Picosecond dynamics of primary electron-transfer processes in bacterial photosynthesis. Biophys J. 1978 Aug;23(2):207–217. doi: 10.1016/S0006-3495(78)85443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockley M. G., Windsor M. W., Cogdell R. J., Parson W. W. Picosecond detection of an intermediate in the photochemical reaction of bacterial photosynthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2251–2255. doi: 10.1073/pnas.72.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarai A. Possible role of protein in photosynthetic electron transfer. Biochim Biophys Acta. 1980 Jan 4;589(1):71–83. doi: 10.1016/0005-2728(80)90133-4. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Parson W. W. Energies and kinetics of radical pairs involving bacteriochlorophyll and bacteriopheophytin in bacterial reaction centers. Proc Natl Acad Sci U S A. 1981 Feb;78(2):957–961. doi: 10.1073/pnas.78.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A. Role of the chlorophyll dimer in bacterial photosynthesis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3105–3109. doi: 10.1073/pnas.77.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraight C. A. Electron acceptors of bacterial photosynthetic reaction centers. II. H+ binding coupled to secondary electron transfer in the quinone acceptor complex. Biochim Biophys Acta. 1979 Nov 8;548(2):309–327. doi: 10.1016/0005-2728(79)90138-5. [DOI] [PubMed] [Google Scholar]


