Abstract
The dimensions of bacteriophage T7 and T7 capsids have been investigated by small-angle x-ray scattering. Phage T7 behaves like a sphere of uniform density with an outer radius of 301 +/- 2 A (excluding the phage tail) and a calculated volume for protein plus nucleic acid of 1.14 +/- 0.05 x 10(-16) ml. The outer radius determined for T7 phage in solution is approximately 30% greater than the radius measured from electron micrographs, which indicates that considerable shrinkage occurs during preparation for electron microscopy. Capsids that have a phagelike envelope and do not contain DNA were obtained from lysates of T7-infected Escherichia coli (capsid II) and by separating the capsid component of T7 phage from the phage DNA by means of temperature shock (capsid IV). In both cases the peak protein density is at a radius of 275 A; the outer radius is 286 +/- 4 A, approximately 5% smaller than the envelope of T7 phage. The thickness of the envelope of capsid II is 22 +/- 4 A, consistent with the thickness of protein estimated to be 23 +/- 5 A in whole T7 phage, as seen on electron micrographs in which the internal DNA is positively stained. The volume in T7 phage available to package DNA is estimated to be 9.2 +/- 0.4 x 10(-17) ml. The packaged DNA adopts a regular packing with 23.6 A interplanar spacing between, DNA strands. The angular width of the 23.6 A reflection shows that the mean DNA-DNA spacing throughout the phage head is 27.5 +/- less than 2.2 A. A T7 precursor capsid (capsid I) expands when pelleted for x-ray scattering in the ultracentrifuge to essentially the same outer dimensions as for capsids II and IV. This expansion of capsid I can be prevented by fixing with glutaraldehyde; fixed capsid I has peak density at a radius of 247 A, 10% less than capsid II or IV.
Full text
PDF


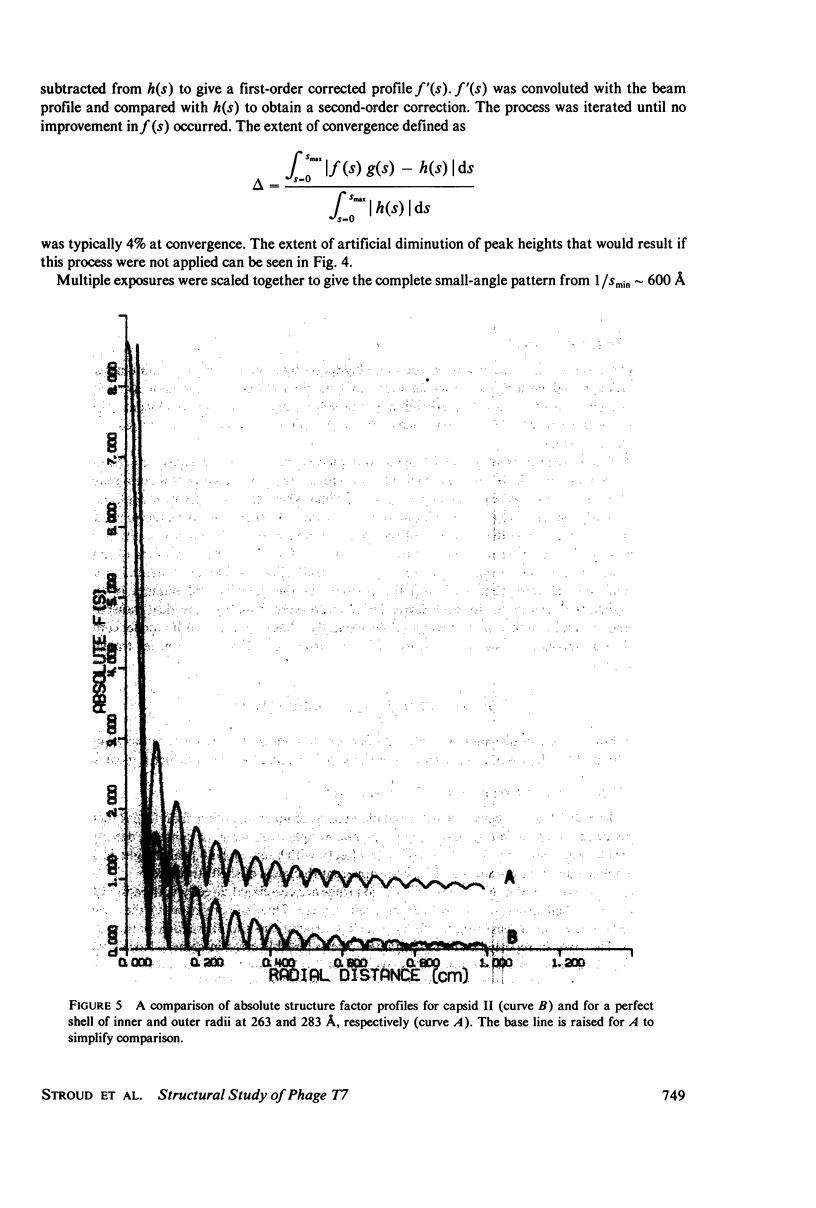

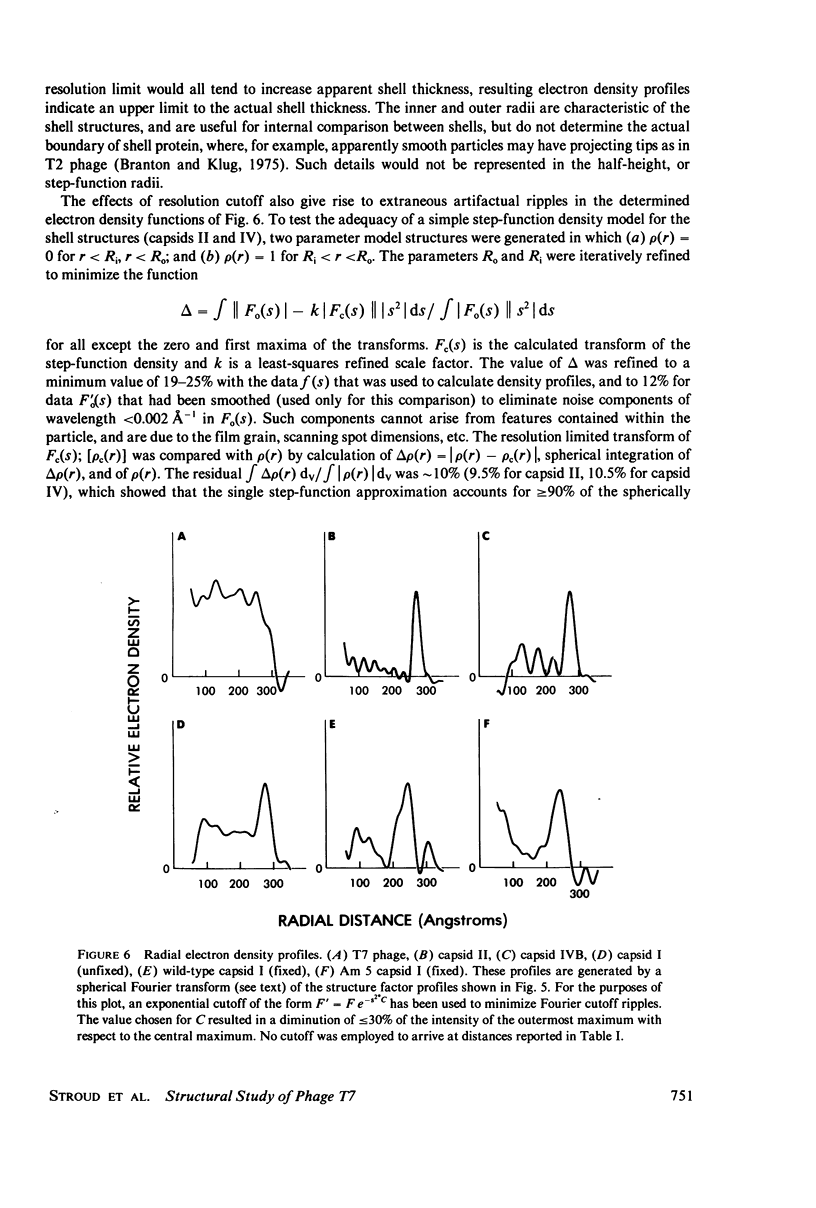
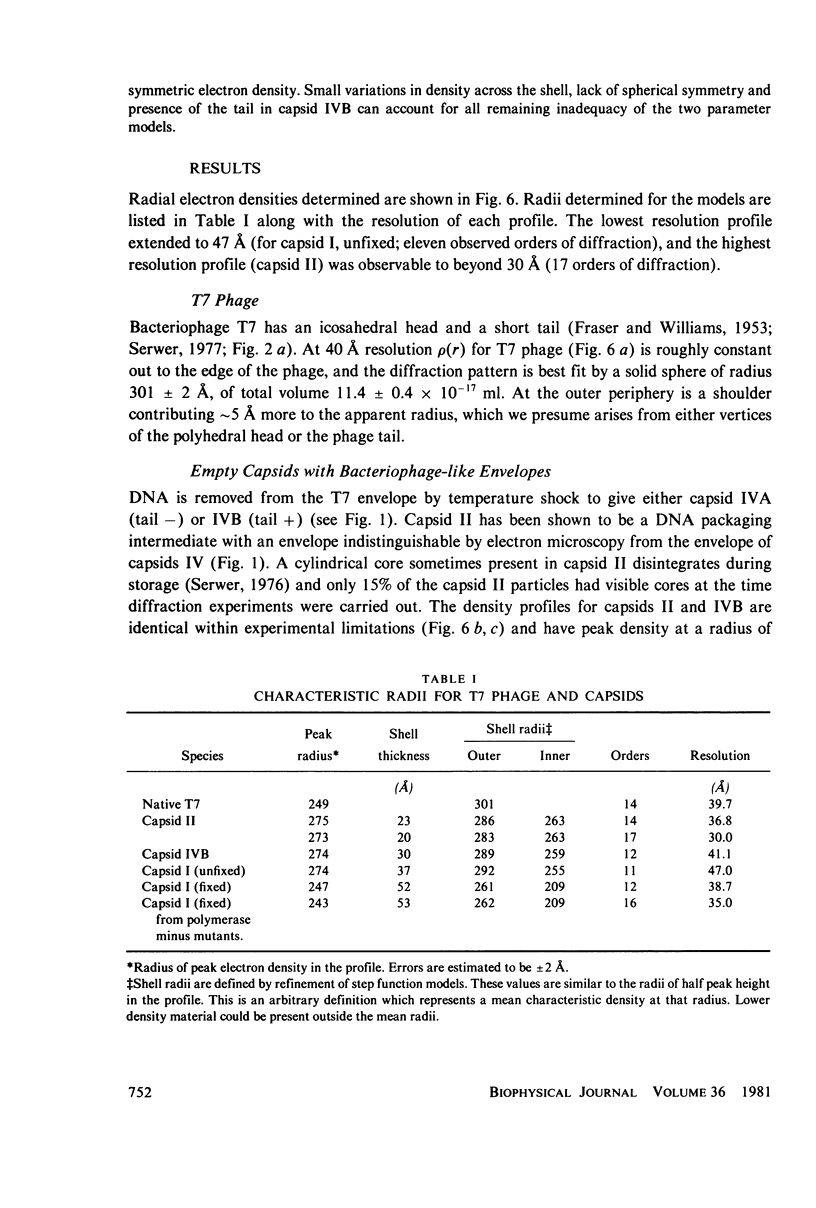





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDEREGG J. W., GEIL P. H., BEEMAN W. W., KAESBERG P. An x-ray scattering investigation of wild cucumber mosaic virus and a related protein. Biophys J. 1961 Nov;1:657–667. doi: 10.1016/s0006-3495(61)86915-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDEREGG J. W., WRIGHT M., KAESBERG P. An x-ray scattering study of bromegrass mosaic virus. Biophys J. 1963 Mar;3:175–182. doi: 10.1016/s0006-3495(63)86813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler S., Modrich P. T7-induced DNA polymerase. Characterization of associated exonuclease activities and resolution into biologically active subunits. J Biol Chem. 1979 Nov 25;254(22):11605–11614. [PubMed] [Google Scholar]
- Bancroft F. C., Freifelder D. Molecular weights of coliphages and coliphage DNA. I. Measurement of the molecular weight of bacteriophage T7 by high-speed equilibrium centrifugation. J Mol Biol. 1970 Dec 28;54(3):537–546. doi: 10.1016/0022-2836(70)90124-5. [DOI] [PubMed] [Google Scholar]
- Branton D., Klug A. Capsid geometry of bacteriophage T2: a freeze-etching study. J Mol Biol. 1975 Mar 15;92(4):559–565. doi: 10.1016/0022-2836(75)90309-5. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Harrison S. C. DNA arrangement in isometric phage heads. Nature. 1977 Aug 18;268(5621):598–602. doi: 10.1038/268598a0. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Hendrix R. W., King J. Structural studies of bacteriophage lambda heads and proheads by small angle X-ray diffraction. J Mol Biol. 1979 Nov 5;134(3):575–594. doi: 10.1016/0022-2836(79)90368-1. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., King J., Eiserling F. A. The size of the bacteriophage T4 head in solution with comments about the dimension of virus particles as visualized by electron microscopy. J Mol Biol. 1978 Jun 25;122(2):247–253. doi: 10.1016/0022-2836(78)90040-2. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., King J., Harrison S. C., Eiserling F. A. The structural organization of DNA packaged within the heads of T4 wild-type, isometric and giant bacteriophages. Cell. 1978 Jul;14(3):559–568. doi: 10.1016/0092-8674(78)90242-8. [DOI] [PubMed] [Google Scholar]
- Earnshaw W., Casjens S., Harrison S. C. Assembly of the head of bacteriophage P22: x-ray diffraction from heads, proheads and related structures. J Mol Biol. 1976 Jun 25;104(2):387–410. doi: 10.1016/0022-2836(76)90278-3. [DOI] [PubMed] [Google Scholar]
- FRASER D., WILLIAMS R. C. Details of frozen-dried T3 and T7 bacteriophages as shown by electron microscopy. J Bacteriol. 1953 Feb;65(2):167–170. doi: 10.1128/jb.65.2.167-170.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni G., Padden F. J., Jr, Keith H. D. Crystallization of DNA from dilute solution. Proc Natl Acad Sci U S A. 1969 Mar;62(3):964–971. doi: 10.1073/pnas.62.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C. Structure of tomato bushy stunt virus. I. The spherically averaged electron density. J Mol Biol. 1969 Jun 28;42(3):457–483. doi: 10.1016/0022-2836(69)90236-8. [DOI] [PubMed] [Google Scholar]
- Hori K., Mark D. F., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. Purification and properties of the phage-encoded subunit, the gene 5 protein. J Biol Chem. 1979 Nov 25;254(22):11591–11597. [PubMed] [Google Scholar]
- Jack A., Harrison S. C. On the interpretation of small-angle x-ray solution scattering from spherical viruses. J Mol Biol. 1975 Nov 25;99(1):15–25. doi: 10.1016/s0022-2836(75)80155-0. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J. Electron microscopical studies of phage multiplication. II. Production of phage-related structures during multiplication of phages T2 and T4. Virology. 1957 Apr;3(2):256–274. doi: 10.1016/0042-6822(57)90092-2. [DOI] [PubMed] [Google Scholar]
- Kistler J., Aebi U., Onorato L., ten Heggeler B., Showe M. K. Structural changes during the transformation of bacteriophage T4 polyheads: characterization of the initial and final states by freeze-drying and shadowing Fab-fragment-labelled preparations. J Mol Biol. 1978 Dec 15;126(3):571–589. doi: 10.1016/0022-2836(78)90059-1. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., PRATT E. A. On the structure of the glucosylated hydroxymethylcytosine nucleotides of coliphages T2, T4, and T6. J Biol Chem. 1960 Nov;235:3254–3259. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lerman L. S., Wilkerson L. S., Venable J. H., Jr, Robinson B. H. DNA packing in single crystals inferred from freeze-fracture-etch replicas. J Mol Biol. 1976 Dec;108(2):271–293. doi: 10.1016/s0022-2836(76)80121-0. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Becker A. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiol Rev. 1978 Sep;42(3):529–576. doi: 10.1128/mr.42.3.529-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH A. C., RICH A. X-ray diffraction studies of bacterial viruses. Nature. 1961 Sep 23;191:1242–1245. doi: 10.1038/1911242a0. [DOI] [PubMed] [Google Scholar]
- Richards K. E., Williams R. C., Calendar R. Mode of DNA packing within bacteriophage heads. J Mol Biol. 1973 Aug 5;78(2):255–259. doi: 10.1016/0022-2836(73)90114-9. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Sadowski P. D. Bacteriophage T7 morphogenesis: phage-related particles in cells infected with wild-type and mutant T7 phage. Virology. 1977 Jan;76(1):263–285. doi: 10.1016/0042-6822(77)90302-6. [DOI] [PubMed] [Google Scholar]
- Serwer P. A metrizamide-impermeable capsid in the DNA packaging pathway of bacteriophage T7. J Mol Biol. 1980 Mar 25;138(1):65–91. doi: 10.1016/s0022-2836(80)80005-2. [DOI] [PubMed] [Google Scholar]
- Serwer P. Buoyant density sedimentation of macromolecules in sodium iothalamate density gradients. J Mol Biol. 1975 Mar 5;92(3):433–448. doi: 10.1016/0022-2836(75)90290-9. [DOI] [PubMed] [Google Scholar]
- Serwer P. Flattening and shrinkage of bacteriophage T7 after preparation for electron microscopy by negative staining. J Ultrastruct Res. 1977 Mar;58(3):235–243. doi: 10.1016/s0022-5320(77)90015-6. [DOI] [PubMed] [Google Scholar]
- Serwer P. Internal proteins of bacteriophage T7. J Mol Biol. 1976 Nov 5;107(3):271–291. doi: 10.1016/s0022-2836(76)80005-8. [DOI] [PubMed] [Google Scholar]
- Serwer P., Watson R. H. Capsid-DNA complexes in the DNA packaging pathway of bacteriophage T7: characterization of the capsids bound to monomeric and concatemeric DNA. Virology. 1981 Jan 15;108(1):164–176. doi: 10.1016/0042-6822(81)90536-5. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Couture E., Aebi U., Showe M. K. Structure of T4 polyheads. II. A pathway of polyhead transformation as a model for T4 capsid maturation. J Mol Biol. 1976 Sep 5;106(1):187–221. doi: 10.1016/0022-2836(76)90307-7. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Subirana J. A., Lloveras J., Lombardero M., Viñuela E. X-ray scattering of the non-isometric Bacillus subtilis phage phi29. J Mol Biol. 1979 Feb 15;128(1):101–106. doi: 10.1016/0022-2836(79)90310-3. [DOI] [PubMed] [Google Scholar]
- Tikchonenko T. I. Conformation of viral nucleic acids in situ. Adv Virus Res. 1969;15:201–290. doi: 10.1016/s0065-3527(08)60876-3. [DOI] [PubMed] [Google Scholar]