Abstract
A rapid zidovudine (ZDV) resistance genotypic assay was developed based on the mutagenically separated PCR (MS-PCR) technique to detect two ZDV-resistant mutations, M41L and K70R in CRF01_AE (subtype E). Endpoint dilution analysis revealed that the newly constructed MS-PCR assay could successfully detect three to nine copies of human immunodeficiency virus type 1 template RNA. The test against wild-type and mutant template mixtures in different ratios demonstrated that the assay could detect 10% minor population, at least. Fifty-one subtype E clinical samples were analyzed by the newly constructed MS-PCR assay and direct nucleotide sequencing. The concordance of the two assays was 92 and 100% in codons 41 and 70, respectively. The MS-PCR assay is a rapid, simple, and inexpensive assay that is highly sensitive in detecting mutant targets, including minor populations. Thus, it could be used as a powerful tool for epidemiological surveillance of drug-resistant mutations in developing countries.
Southeast Asia has the second highest human immunodeficiency virus type 1 (HIV-1) prevalence in the world (29). In this area, antiretroviral therapy (ARV) had been available for only limited numbers of patients (28, 30). In Thailand, zidovudine (ZDV) prophylaxis has been widely available for the prevention of mother-to-child transmission. The recent price reduction by drug companies has highlighted the extent of this problem to HIV-1-infected people living in the area. Increased availability and usage of ARV will increase the emergence of drug-resistant HIV-1. Therefore, it is important to monitor the future spread of drug-resistant HIV-1. Studies done in Europe and the United States have shown up to 15% of primary HIV-1 infection with drug-resistant viruses (5, 18). This transmission of drug-resistant virus may become an important public health problem in choosing the effective treatment (8) and an international surveillance program to monitor HIV-1 drug resistance in treatment-naive patients has also been proposed (5).
Drug-resistant genotyping by the standard nucleotide sequencing method costs $200 to $500 (19) and necessitates the use of expensive equipment, i.e., an autosequencer. Therefore, the availability of the genotyping by standard nucleotide sequencing is very limited in developing countries. Other available genotyping assays, such as the line probe assay (LiPA), selective PCR (also called ARMS [for amplification refractory mutation detection system]), the oligonucleotide ligation assay (OLA), and the heteroduplex tracking assay (HTA), are available but lower sensitivity, lower specificity, and complicated procedures limits their usage (5, 21, 27, 31, 32). To overcome the problem, less-expensive simple and sensitive genotyping technology is urgently required. We have previously demonstrated that the principle of mutagenically separated PCR (MS-PCR), first developed by Rust et al. (24), can be applied to the HIV-1 nonnucleoside analogue reverse transcriptase (RT) and protease inhibitor-resistant mutation detection (6).
The M41L, D67R, K70R, T215F/Y, L210W, and K219Q mutations are known to be associated with ZDV resistance in subtype B isolates (7, 10, 17). Similar patterns of nucleoside analogue RT inhibitors resistant mutations were also seen in CRF01_AE (26; K. Ariyoshi et al., unpublished data), the predominant HIV-1 strain in Southeast Asia (15, 20, 25). Among these, M41L and K70R were the most frequent mutations; the K70R RT mutation usually appears in the early phase and M41L appears later, together with other RT mutations associated with resistance (1, 12). In our study, we constructed the MS-PCR assay for the detection of M41L and K70R HIV-1 RT resistance mutations in CRF01_AE, since ZDV is the most common ARV used as single or combination therapy with other antiretroviral drugs.
MATERIALS AND METHODS
Primer design and construction of MS-PCR.
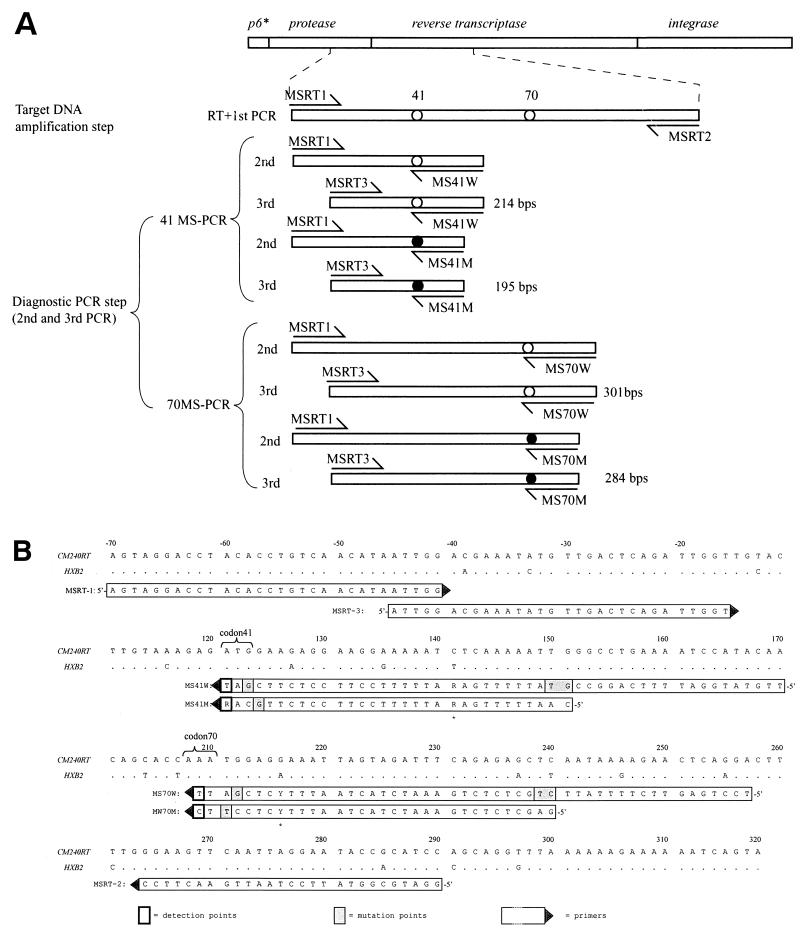
The assay is single nucleotide polymorphism detection PCR that consists of a one-step RT-PCR reaction and two subsequent rounds of competitive PCR. The first-round RT-PCR amplified a DNA fragment, including detection points codon 41 and 70 of RT (Fig. 1A), whereas the subsequent two rounds of PCR constituted a diagnostic competitive PCR to determine nucleotide patterns of the targeted sequence. Two competitive detection primers are required for each detection point. One is for wild-type detection (MS41W and MS70W), and the other is for mutant detection (MS41M and MS70M). The design of the detection primers is key to the success of this assay, and there are three basic rules for the primer design: the 3′ end of each detection primer is designed to correspond to the wild-type or the mutant nucleotide pattern; to distinguish wild-type and mutant amplicons on electrophoresis, wild-type detection primers must be ca. 20 bp longer than the mutant primers. Finally, introduction of a mutation at the second, third, or fourth position from the 3′ end of the detection primers increases the specificity of primer template annealing (2, 6, 24). Applying these rules, additional substitutions were introduced in the third or fourth of the detection primers, as shown in Fig. 1B.
FIG. 1.
(A) Overview of 41- and 70MS-PCRs. Amplified DNA fragments are depicted as bars, arrows indicate primers, and detection points are shown as circles. Open circles and solid circles indicate wild types and mutations, respectively. MSRT1 and MSRT2 are used to amplify the DNA target templates. Subsequent second and third PCR steps were performed independently in 41- and 70MSPCR. As for 41MS-PCR, MSRT1, MS41W, and MS41M primers were used in the second-round PCR, and MSRT3, MS41W, and MS41M primers were used for the third PCR. The final wild-type and mutant amplicons in 41MS-PCR are 214 and 195 bp, respectively. As for 70MS-PCR, MSRT1, MS70W, and MS70M primers were used in the second PCR, and MSRT3, MS70W, and MS70M primers were used for the third PCR. The final wild-type and mutant amplicons in 70MS-PCR are 301 and 284 bp, respectively. (B) The primer sequences used in the assay are shown aligned with reference polymerase sequence CRF01_AE CM240. Nucleotide numbers shown beside the sequence are based on CM240 sequence data from Los Alamos database(accession no. U54771). Primer directions are indicated by solid triangles. Detection points are indicated with bold squares, and artificially introduced mutations are shaded in gray.
MS-PCR.
Viral RNA was extracted from 200 μl of patient plasma by using a commercially available extraction kit (Roche Molecular Biochemicals, Mannheim, Germany). One-step RT-PCR with the MSRT1-MSRT2 primer pair and one-step RNA PCR (AMV) kit (TaKaRa Biomedical, Osaka, Japan) were performed to amplify a 370-bp RT template, including RT codon 41 and 70 target loci.
Primer MSRT1, for the second PCR, and primer MSRT3, for the third PCR, were used together with primers MS41W and MS41M, for 41MS-PCR, and primers MS70W and MS70M, for the 70MS-PCR, respectively. Taq DNA polymerase (Promega) was used in the second and third PCRs. Details of each PCR are summarized in the Table 1. All PCR were performed by GeneAmp PCR system 9700 thermal cycler (PE Applied Biosystems, Foster City, Calif.), and the PCR products were analyzed on 3% agarose gel electrophoresis (NuSieve GTG agarose; BMA, Rockland, Maine) at 120 V for 45 min. The amplicons were visualized under UV light, and the image was recorded.
TABLE 1.
PCR conditions and primers used for the reactions
| Step | PCR program
|
Primers (amt [pmol]) | |
|---|---|---|---|
| Temp (°C), duration | No. of cycles | ||
| RT-PCRa (template amplification) | 55, 30 min | 1 | MSRT1 (12), MSRT2 (12) |
| 95, 2 min | 1 | ||
| 94, 30 s; 58, 30 s; 72, 30 s | 7 | ||
| Second PCRb (detection) | 94, 30 s; 58, 30 s; 72, 30 s | 20 | 41 MS-PCR: MSRT1 (3), MS41W (2), MS41M (3) |
| 70MS-PCR: MSRT1 (5), MS70W (2), MS70M (4) | |||
| Third PCRb (detection) | 94, 30 s; 58, 30 s; 72, 30 s | 40 | 41MS-PCR: MSRT3 (10), MS41W (8), MS41M (20) |
| 70MS-PCR: MSRT3 (10), MS70W (8), MS70M (20) | |||
RT-PCR: 5 mM MgCl2, 1 mM deoxynucleoside triphosphates, 1 × One-Step PCR buffer (Takara).
Second and third PCRs 1mM MgCl2, 0.08 mM deoxynucleoside triphosphates, 1 × PCR buffer (Promega).
Control DNA templates and assay optimization.
CRF01_AE pol gene control DNA templates were prepared to test primer specificity and sensitivity. Four patterns of mutation combinations such as C-1 (41M plus 70K), C-2 (41L plus 70R), C-3 (41M plus 70R), and C-4 (41L plus 70K) were constructed from CRF01__AE-infected patient samples. Patient samples with corresponding mutation combinations were selected by direct sequencing of 1.7-kb pol gene, and then the pol fragments were cloned into the pCR-Blunt II TOPO cloning vector (Invitrogen, Carlsbad, Calif.). The mutation pattern of each clone was verified by sequencing. The 1.7-kb pol gene fragment was excised from the vector, purified by using the QIAquick Gel extraction kit (Qiagen, Inc., Valencia, Calif.) and used as a control DNA template. Sequencing was carried out by using the ABI Prism Big Dye terminator cycle sequencing kit with an ABI Prism 3100 DNA sequencer from PE Biosystems.
Evaluation of assay performance.
To evaluate the detection limit of the assay, plasma samples were selected from three patients with known RNA copy numbers and mutation patterns at codons 41 and 70 as follows: patient 1 (P-1), 8,300 copies/ml, 41L plus 70K; patient 2 (P-2), 1,500 copies/ml, 41M plus 70K; and patient 3 (P-3), 171,000 copies/ml, 41M>L plus 70R (mixture of 41M and -L with a predominance of M). RNA quantification was done with Amplicor HIV-1 monitor test kit (Roche). After viral RNA extraction, serial dilutions of the RNA samples were prepared in distilled water, and each dilution was tested with MS-PCR assays.
To evaluate the sensitivity in the detection of minor virus population, C-1 (41M, 70K) and C-2 (41L, 70R) control DNA templates were mixed in the following ratios: 1:1, 1:10−1, 1:10−2, 1:10−3, 1:10−4, 10−1:1, 10−2:1, 10−3:1, and 10−4:1. The highest DNA amount was 2 pg, which corresponded to 106 copies of the template DNA, and serial 10-fold dilutions were made to 102 copies. The template mixtures were then applied to MS-PCR standard protocol without the first RT step. The studies were performed for the both codon 41 and 70 MS-PCR.
To evaluate assay performance against clinical samples, a total of 51 plasma samples from patients infected with CRF01 were tested: 16 from Japan and 35 from patients attending the Day Care Centre in Lampang hospital in Thailand. The HIV-1 viral load was tested by using the Amplicore HIV-1 monitor test (Roche), and CD4 T cells were measured by flow cytometry.
Direct sequencing of RT and C2V3 Subtyping of clinical samples.
RT-PCR and nested PCR were performed to amplify the RT region. The outer primers DRRT1L (5′-ATG ATA GGG GGA ATT GGA GGT TT) and DRRT4L (5′-TAC TTC TGT TAG TGC TTT GGT TCC) were used in the first One-Step RT-PCR. For the second-round (nested) PCR, the primers used were DRRT7L (5′-GAC CTA CAC CTG TCA ACA TAA TTG G) and DRRT6L (5′-TAA TCC CTG CAT AAA TCT GAC TTG C). Sequencing reactions were carried out with primers from the second round of PCR. The subtype of each sample was determined by sequencing the C2V3 region of the HIV-1 envelope gene (16). In brief, proviral DNA was extracted from patient PBMC, and the env C2V3 region of HIV was amplified. Amplified C2V3 and RT fragments were sequenced by using an ABI 3100 autosequencer (Applied Biosystems), and the ABI Prism Big Dye terminator cycle sequencing ready reaction kit from PE Biosystems. Data were aligned by CLUSTALW program and analyzed by the neighbor-joining method, together with subtype-reference sequences from Los Alamos database.
RESULTS
Discrimination of wild type and mutant at codon 41 and 70.
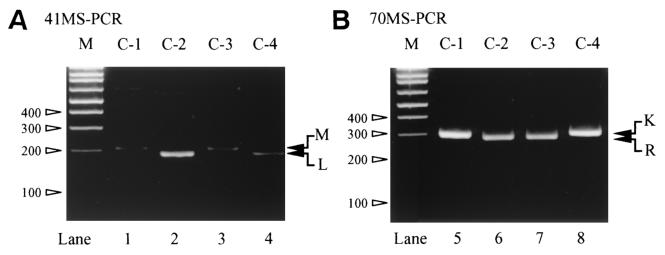
Four control DNA templates, C-1, C-2, C-3, and C-4 were analyzed by the newly constructed MS-PCR for codon 41 (41MS-PCR) and codon 70 (70MS-PCR). As shown in Fig. 2, 41MS-PCR clearly distinguished 41M, which appeared as a 214-bp band (lanes 1 and 3) from 41L, a 195-bp band (lanes 2 and 4). The 70MS-PCR was also effective in distinguishing codon 70K, seen as a 301-bp band (C-1 and C-4; lanes 1 and 4), from 70R, which was seen as a 284-bp band (C-2 and C-3; lanes 2 and 3).
FIG. 2.
Result of the four control templates: C-1 (41M, 70K), C-2 (41L, 70R), C-3(41M, 70R), and C-4 (41M, 70K). “M” on the upper side of each gel image indicate 100-bp DNA ladder marker, and the sizes of representative bands are shown in the left side of the each gel image. (A) 41MS-PCR results. Wild-type (214-bp) and mutant (195-bp) bands are indicated with “M” and “L” with arrows in the right side of the gel image. (B) 70MS-PCR results. Wild-type (301-bp) and mutant (284-bp) bands are indicated with “K” and “R” with arrows in the right side of the gel image.
Sensitivity of the assay.
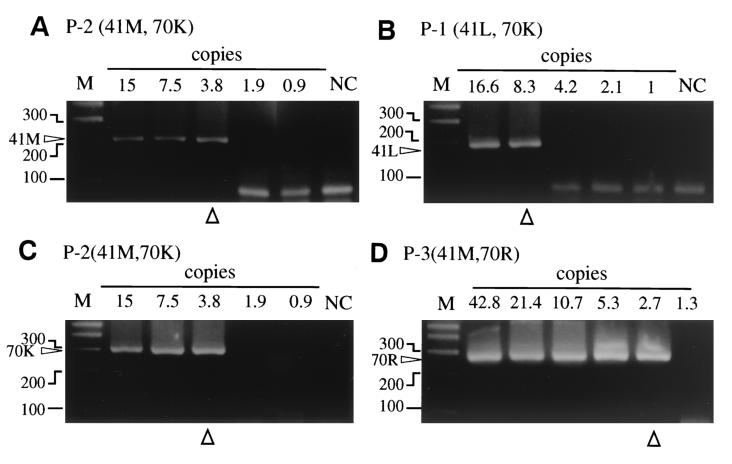
Endpoint detection limits of the MS-PCR assays were enhanced by limiting dilution. The range of RNA copy numbers tested in the patients were 166 to 0.5, 30 to 0.93, and 42.75 to 0.66 copies for P-1, P-2, and P-3, respectively.
The detection limits for the 41M isolates in P-2 and P-3 were 3.8 and 2.7 copies, respectively (Table 2), and for the 41L isolate in P-1 it was 8.3. The detection levels for wild-type (M) and mutant (L) detection in 41MS-PCR were quite similar. The thresholds of detection for the 70K were 8.3 and 3.8 in P-1 and P-2, respectively. Although smears made it rather difficult to determine the real mutant band, the endpoint of 70R was 2.7 in P-3. The endpoint detection of wild-type (K) and mutant (R) detection was also quite similar. In summary, our data show that both MS-PCR assays were sufficiently sensitive to detect virus at fewer than 10 copies. Moreover, Fig. 3 demonstrated all-or-nothing type results in both assays. To confirm the detection limit of the assay, we also performed limiting dilution assays of the patients' plasma samples with normal human plasma as a diluent. The results were consistent with our previous results, and the limit was 10 copies (data not shown).
TABLE 2.
Detection endpoints of 41- and 70MS-PCRs
| Codon | No. of copies per reactiona
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a/a | P-1(41L, 70K)
|
P-2(41M, 70K)
|
P-3(41M>L, 70R)c
|
||||||||||||||||||
| 166 (1×) | 16.6 (10×) | 8.3b (20×) | 4.2 (40×) | 2.1 (80×) | 1 (160×) | 0.5 (320×) | 30 (1×) | 15 (2×) | 7.5 (4×) | 3.8b (8×) | 1.9 (16×) | 0.9 (32×) | 42.8 (80×) | 21.4 (160×) | 10.7 (320×) | 5.3 (640×) | 2.7b (1,280×) | 1.3 (2,560×) | 0.7 (5,120×) | ||
| 41 | M | − | − | − | − | − | − | − | + | + | + | + | − | − | + | + | + | + | + | − | − |
| L | + | + | + | − | − | − | − | − | − | − | − | − | − | + | + | − | − | − | − | − | |
| 70 | K | + | + | + | − | − | − | − | + | + | + | + | − | − | − | − | − | − | − | − | − |
| R | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + | − | − | |
“1×”, etc., indicates the number of dilutions.
Endpoint detection limit of the assay.
The 41L variant is the minor population.
FIG. 3.
Representative result of limiting dilution assay to evaluate detection limits of 41 and 70MSPCR. Numbers on the upper side of each gel give the number of HIV-1 RNA copies in each reaction. “M” on the upper side indicates a 100-bp DNA ladder marker, and the sizes of representative bands are shown in the left side of the each gel image. The endpoint of each assay is marked with open triangle in the bottom of the gel image. (A) Wild-type detection with 41MSPCR. Amplified bands are indicated with “41M” in the left side of the gel image. (B) Mutant detection with 41MSPCR. Amplified bands are indicated with “41L” in the left side of the gel image. (C) Wild-type detection with 70MSPCR. Amplified bands are indicated with “70K” in the left side of the gel image. (D) Mutant detection with 70MSPCR. Amplified bands are indicated with “70R” in the left side of the gel image.
Assay sensitivity to detect minor virus population.
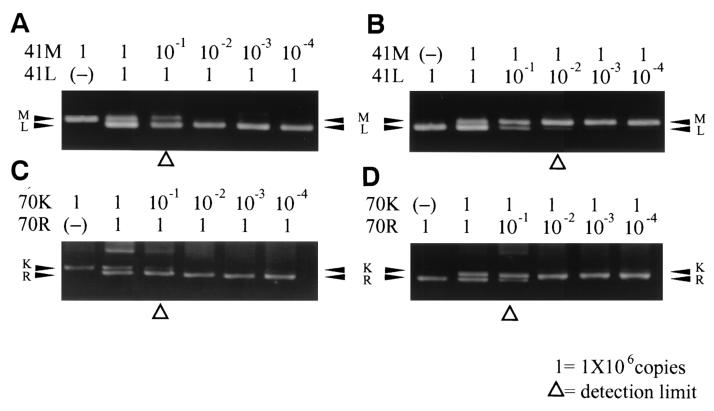
By fixing the C-1 wild-type template by reducing the amount of C-2 mutant template in the 41MS-PCR assay, minor mutant bands up to a 1:10 ratio could be detected clearly, and we could also identify a faint band in the 1:100 ratio. By fixing the C-2 template and reducing the C-1 template, the minor wild-type band was seen at dilutions of up to 1:10 (Fig. 4). However, with mixed-template result of 70MS-PCR revealed only a 1:10 detection rate of minor variant for the wild type mixed with mutant in any position. Overall, the MS-PCR assays were sensitive enough to detect at least a 1:10 ratio of both wild-type and mutant mixed templates.
FIG. 4.
MS-PCR results of the mixed control templates. The relative amount of the templates is shown on the upper side of each gel. “1” represents ca. 106 copies of DNA templates. Wild-type and mutant bands are indicated with corresponding amino acid codes and arrows in the left side of the each gel. The detection limit is highlighted with an open triangle in the each gel. (A) Detection limits of 41M mixed with 41L. (B) Detection limits of 41L mixed with 41M. (C) Detection limits of 70K mixed with 70R. (D) Detection limits of 70R mixed with 70K.
Testing MS-PCR on patient samples.
The results of individual cases are shown in Table 3 and include treatment, viral load, and CD4-positive T-cell count at the time of sample collection. Among 51 patients, 19 had been treated with a regimen that included ZDV, and 9 had ZDV-resistant mutations. There were also five patients with histories of previous ZDV administration in the enrolled samples, and none of these samples had drug-resistant mutations.
TABLE 3.
Summary of enrolled sample profile and results of the assay
| Patient profile | Sequence resultg as determined by:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Treatment (durationf) | Viral load (log) | CD4 (mm3) | Direct sequence
|
MS-PCR
|
||||||
| M41 | D67 | K70 | L210 | T215 | K219 | M41 | K70 | ||||
| 1 | ZDV + ddC (2.3Y) | 4.6 | 223 | M | N | R | L | I | Q | M | R |
| 2 | Naive | 4.1 | 404 | M | D | K | L | T | K | M | K |
| 3 | Naive | 4.6 | 565 | M | D | K | L | T | K | M | K |
| 4 | Naive | 5.9 | 165 | M | D | K | L | T | K | M | K |
| 5 | Past ZDVa | 5.2 | 90 | M | D | K | M | T | K | M | K |
| 6 | Naive | 5.9 | 0 | M | D | K | L | T | K | M | K |
| 7 | Naive | 4.2 | 371 | M | D | K | L | T | K | M | K |
| 8 | Naive | 5.6 | 284 | M | D | K | L | T | K | M | K |
| 9 | ZDV + 3TC + NVP (1M) | 2.6 | 127 | M | D | K | L | T | K | M/L | Kh |
| 10 | Naive | 4.8 | 306 | M | D | K | L | T | K | M | K |
| 11 | Naive | 5.5 | 226 | M | D | K | L | T | K | M | K |
| 12 | Naive | 6.0 | 61 | M | D | K | L | T | K | M | K |
| 13 | ZDV + ddI (2Y) | 5.3 | 329 | M | D | K | L | T | K | M | K |
| 14 | ZDV + 3TC + NVP (2M) | 2.6 | 106 | M | D | K | L | T | K | M | K |
| 15 | Naive | 3.9 | 441 | M | D | K | L | T | K | M | K |
| 16 | Naive | 5.2 | 869 | M | D | K | L | T | K | M | K |
| 17 | Naive | 5.5 | 0 | M | D | K | L | T | K | M | K |
| 18 | Past ZDVb | 4.9 | 607 | M | D | K | L | T | K | M | K |
| 19 | Naive | 6.0 | 20 | M | D | K | L | T | K | M | K |
| 20 | Naive | 5.5 | 133 | M | D | K | L | T | K | M | K |
| 21 | Past ZDV + ddIc | 4.2 | 389 | M | D | K | L | T | K | M | K |
| 22 | Naive | 4.5 | 416 | M | D | K | L | T | K | M | K |
| 23 | ZDV + ddC (6M) | 5.1 | 489 | M | D | R | L | Y | Q | M/L | Rh |
| 24 | ZDV + ddI/ddC (7M) | 5.3 | 321 | M | D | K | L | T | K | M | K |
| 25 | Naive | 4.6 | 556 | M | D | K | L | T | K | M | K |
| 26 | Naive | 3.4 | 531 | M | D | K | L | T | K | M | K |
| 27 | Naive | 4.2 | 224 | M | D | K | L | T | K | M | K |
| 28 | Naive | 4.1 | 297 | M | D | K | L | T | K | M | K |
| 29 | Naive | 5.8 | 41 | M | D | K | L | T | K | M | K |
| 30 | Naive | 6.2 | 29 | M | D | K | L | T | K | M | K |
| 31 | Naive | 4.5 | 415 | M | D | K | L | T | K | M | K |
| 32 | Naive | 5.4 | 177 | M | D | K | L | T | K | M | K |
| 33 | Past ZDVd | 4.8 | 45 | M | D | K | L | T | K | M | K |
| 34 | Naive | 5.2 | 69 | M | D | K | L | T | K | M | K |
| 35 | Naive | 5.5 | 44 | M | D | K | L | T | K | M | K |
| 36 | ZDV + 3TC/ddI (2Y) | 3.6 | 271 | M | D | K | L | T | K | M | K |
| 37 | ZDV/3TC + d4T (2Y) | 5.5 | 117 | M/L | N | R | L | F | Q | M/L | R |
| 38 | ZDV + 3TC/+ddI + d4T (4Y) | 5.6 | 8 | M | N | R | W | F | K | M | R |
| 39 | ZDV + ddI/ddC (3.5Y) | 3.9 | 6 | L | N | K | W | Y | N | L | K |
| 40 | ZDV + ddI/ddC/3TC (3Y) | 5.1 | 13 | M | D | K | L | I | K | M | K |
| 41 | ZDV + 3TC (3Y) | 3.9 | 373 | M | D | K | L | T | K | M | K |
| 42 | ZDV + ddI/3TC (2.5Y) | 4.6 | 49 | L | N | K | W | Y | K | M/L | Kh |
| 43 | ZDV + 3TC (10M) | 5.5 | 9 | M | D | K | L | T | K | M | K |
| 44 | ZDV + 3TC (0.5M) | 4.7 | 55 | M | D | K | L | T | K | M | K |
| 45 | ZDV + ddC/3TC (2.7Y) | 4.6 | 76 | L | N | R | L | F | Q | L | R |
| 46 | Past ZDV + ddC/3TCc | 4.7 | 1 | M | D | K | L | T | K | M | K |
| 47 | ZDV/+ ddC (4.5M) | 5.2 | 0 | M | N | R | L | F | K | M/L | Rh |
| 48 | Naive | 3.1 | 509 | M | D | K | L | T | K | M | K |
| 49 | Naive | 5.8 | 78 | M | D | K | L | T | K | M | K |
| 50 | ZDV + ddC (1.5M) | 2.6 | NDi | M | D | K | L | T | K | M | K |
| 51 | ZDV + ddI/ddC (2.5Y) | 4.6 | 27 | L | N | K | W | Y | K | L | K |
History of 1 months of ZDV monotherapy 2 years ago.
History of 2 months of ZDV monotherapy 2 years ago.
History of 4 months of ZDV + ddI dual therapy 2 years ago.
History of 1 month of ZDV monotherapy 2 years ago.
History of 1.4 years of ZDV + ddI/3TC dual therapy 1 year ago.
M, months; Y, years.
Mutants are indicated in boldface.
This case had discordant results between direct sequencing and MS-PCR
ND, no data.
The comparison of MS-PCR and the direct sequencing results are summarized in Table 4. As for codon 41, wild type (M) was detected in 46 samples, mutant (L) was detected in 4 samples, and one mixed case (M/L) was detected by the direct sequencing, whereas 43M, 3L, and 5M/L were defined by 41MS-PCR. Comparing the two assays, 47 of 51 samples (92%) were concordant with 43 wild types, 3 mutants, and 1 mixed type (Table 4). Since the assay confirmed all of the mutant samples previously sequenced, the sensitivity of the mutant 41L detected was 100%. For codon 70, sequencing and MS-PCR assay results were consistent with each other for 45 wild types (K) and 6 mutants (R) (Table 4).
TABLE 4.
Comparison between sequencing and MS-PCR results in 51 CRF01 patientsa
| Sequence | Codon 41 sequence results (no. of samples)
|
Sequence | Codon 70 sequence results (no. of samples)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| M | L | M/L | Total | M | L | M/L | Total | ||
| M | 43 | 0 | 0 | 43 | K | 45 | 0 | 0 | 45 |
| L | 0 | 3 | 0 | 3 | R | 0 | 6 | 0 | 6 |
| M/L | 3 | 1 | 1 | 5 | K/R | 0 | 0 | 0 | 0 |
| Total | 46 | 4 | 1 | 51 | Total | 45 | 6 | 0 | 51 |
Values in boldface represent discordant samples.
Five cases of wild-type-mutant mixed results were seen with 41MS-PCR, whereas only one was seen with sequencing. The discrepancies were observed in four cases: 41M in three cases (patients 9, 23, and 47) and 41L in one case (patient 42) by direct sequencing, but the M/L mixture was observed by 41MS-PCR. Two of the mixed results of MS-PCR showed prominent 41M bands that were compatible with sequencing results. The other two discordant cases showed equal intensity of 41M and 41L bands by MS-PCR. The sample that showed a wild-type-mutant mixed result obtained by sequencing showed an equal intensity of wild-type and mutant bands by MS-PCR.
DISCUSSION
Simplified drug resistance genotypic assays such as selective PCR (ARMS), HTA, LiPA, and OLA are available for HIV-1 drug resistance genotyping (5, 21, 27, 31, 32). ARMS is a PCR-based point mutation assay in which wild-type and mutant detection tests are performed in individual reaction tubes. Since there is no competition between the two detection primers as in MS-PCR, ARMS consequently suffers from lower specificity. HTA, LiPA, and OLA require multiple post-PCR steps to obtain the results. Further, the polymorphisms in the probe-target region are critical in these assays, and unexpected mutations in the targeted region may cause false results (4, 13, 21, 23, 32). Commonly, PCR-based assays have this same limitation for the detection of drug-resistant mutations.
To preserve simplicity and to increase sensitivity and specificity, two improvements were introduced in primers of MS-PCR. Designing the detection primer length difference ca. 20 bp and introduction of intentional mismatches to increase the specificity of the assay are the main features of MS-PCR. We found that introducing a mutation at the third or fourth position from the 3′-end detection primers gave better results than introducing a mutation at the second or third positions (data not shown); similar findings were demonstrated earlier (6).
Initially, we attempted to construct the assay in two nested rounds of PCR, but we did not get acceptable results from some of the clinical samples. Unexpected extra bands (DNA smears), which often made it difficult to define the results, were observed in the two-round nested PCR protocol. In the process of verification of the assay condition, we found that the addition of another short-round outer PCR (seven cycles) improved the reliability of the assay results greatly. These modifications have successfully improved the sensitivity and specificity of the PCR-based genotypic assay. Since the assay has three rounds of PCR, standard PCR protocols should be followed strictly to avoid contamination of samples.
For screening purposes, we chose to examine codons 41 and 70 as targets of ZDV-resistant viruses, rather than choosing codon 215, because the genotypic data analyses of previous studies (3, 10, 11, 14, 22) reveal that all of the cases with ZDV-resistant mutations contained at least one mutation at either codon 41 or codon 70. Indeed, in the present study we identified all of the ZDV-resistant viruses by looking at only codons 41 and 70. Thus, by implication, our assay identifies the majority of the ZDV-resistant viruses.
Discordant results between sequencing and MS-PCR methods were seen in four cases (Tables 3 and 4). Of these, three cases had histories of ZDV treatment, and two had drug-resistant mutations: K70R and T215F/Y. Therefore, the discordant result is most probably due to the higher sensitivity in detecting minor population of mutant variant by MS-PCR. With sequencing method, we found nine patients that had at least one ZDV resistance mutation. All of these patients had either 41L or 70R mutations, which were identified by the MS-PCR. Thus, 41- and 70MS-PCR assays alone could detect the majority of ZDV-resistant viruses and the combination of 41- and 70MS-PCR can be applied to a large-scale epidemiological survey.
The International AIDS Society USA Panel has recommended that drug-resistant genotyping should be incorporated into patient management prior to initiation of therapy to guide the choice of new regimens after treatment failure and for guiding therapy for pregnant women (9). However, a direct sequencing method can detect the minor population only up to 25 to 50%, whereas MS-PCR could detect up to 10% of minor population. Drug-resistant variants may persist as minor variants, which might not be detected by sequencing methods but could emerge rapidly and become a major population when antiretroviral therapy is initiated (33). Therefore, it is important to use a sensitive assay such as MS-PCR for the early detection of minor drug-resistant variants. The result of the MS-PCR is a simple “plus” or “minus” of the target locus that is less informative compared to sequencing. However, if we use a suitable set of MS-PCR assays that can detect the required genotypic information, it could provide a better guide for selection of the optimal ARV.
In conclusion, the developed 41- and 70MS-PCR assays are simple, cheap and rapid ZDV resistance genotyping assays that are excellent for detecting ZDV-resistant mutations.
Acknowledgments
This study was supported by the grant from Organization of Pharmaceutical Safety and Research of Japan.
We thank clinicians of the Research Committee on Prevention of Developing Illnesses and Therapy for HIV-Infected Patients of Japan. We also acknowledge the JICA-NIH Project in Thailand and the Medical Research Council for their collaboration and support. J.N.W. is supported by the Wellcome Trust.
REFERENCES
- 1.Boucher, C. A., E. O'Sullivan, J. W. Mulder, C. Ramautarsing, P. Kellam, G. Darby, J. M. Lange, J. Goudsmit, and B. A. Larder. 1992. Ordered appearance of zidovudine resistance mutations during treatment of 18 human immunodeficiency virus-positive subjects. J. Infect. Dis. 165:105-110. [DOI] [PubMed] [Google Scholar]
- 2.Chang, J. G., J.-M. Lu, J.-M. Huang, J.-T. Chen, H.-J. Liu, and C.-P. Chang. 1995. Rapid diagnosis of β-thalassaemia by mutagenically separated polymerase chain reaction (MS-PCR) and its application to prenatal diagnosis. Br. J. Haematol. 91:602-607. [DOI] [PubMed] [Google Scholar]
- 3.Deeks, S. G., N. S. Hellmann, R. M. Grant, N. T. Parkin, C. J. Petropoulos, M. Becker, W. Symonds, M. Chesney, and P. A. Volberding. 1999. Novel four-drug salvage treatment regimens after failure of a human immunodeficiency virus type 1 protease inhibitor-containing regimen: antiviral activity and correlation of baseline phenotypic drug susceptibility with virologic outcome. J. Infect. Dis. 179:1375-1381. [DOI] [PubMed] [Google Scholar]
- 4.Edelstein, R. E., D. A. Nickerson, V. O. Tobe, L. A. Manns-Arcuino, and L. M. Frenkel. 1998. Oligonucleotide ligation assay for detecting mutations in the human immunodeficiency virus type 1 pol gene that are associated with resistance to zidovudine, didanosine, and lamivudine. J. Clin. Microbiol. 36:569-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontaine, E., C. Lambert, J. Servais, D. Ninove, J.-M. Plesseria, T. Staub, V. Arendt, P. Kirpach, I. Robert, F. Schneider, R. Hemmer, and J.-C. Schmit. 1998. Fast genotypic detection of drug resistance mutations in the HIV-1 reverse transcriptase gene of treatment-naive patients. J. Hum. Virol. 1:451-456. [PubMed] [Google Scholar]
- 6.Frater, A. J., C. C. Chaput, S. Beddows, J. N. Weber, and M. O. McClure. 2001. Simple detection of point mutations associated with HIV-1 drug resistance. J. Virol. Methods 93:145-156. [DOI] [PubMed] [Google Scholar]
- 7.Harrigan, P. R., I. Kinghorn, S. Bloor, S. D. Kemp, I. Najera, A. Kohli, and B. A. Larder. 1996. Significance of amino acid variation at human immunodeficiency virus type 1 reverse transcriptase residue 210 for zidovudine susceptibility. J. Virol. 70:593-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht, F. M., R. M. Grant, C. J. Petropoulos, B. Dillon, M. A. Chesney, H. Tian, N. S. Hellmann, N. I. Bandrapalli, L. Digilio, B. Branson, and J. O. Kahn. 1998. Sexual transmission of an HIV-1 variant resistant to multiple reverse transcriptase and protease inhibitors. N. Engl. J. Med. 30:307-311. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch, M. S., F. Brun-Vezinet, R. T. D'Aquila, S. M. Hammer, V. A. Johnson, D. R. Kuritzkes, C. Loveday, J. W. Mellors, B. Clotet, B. Conway, L. M. Demeter, S. Vella, D. M. Jacobsen, and D. D. Richman. 2000. Antiretroviral drug-resistant testing in adults with HIV infection. JAMA 283:2417-2426. [DOI] [PubMed] [Google Scholar]
- 10.Hooker, D., G. Tachedjian, A. E. Soloman, A. D. Gurusinghe, S. Land, C. Birch, J. L. Anderson, B. M. Roy, E. Arnold, and N. J. Deacon. 1996. An in vivo mutation from leucine to tryptophan at position 210 in human immunodeficiency virus type 1 reverse transcriptase contributes to high-level resistance to 3′-azido-3′-deoxythymidine. J. Virol. 70:8010-8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann, G. R., K. Suzuki, P. Cunningham, M. Mukaide, M. Kondo, M. Imai, J. Zaunders, and D. A. Cooper. 2001. Impact of HIV type 1 protease, reverse transcriptase, cleavage site, and p6 mutations on the virological response to quadruple therapy with saquinavir, ritonavir, and two nucleoside analogs. AIDS Res. Hum. Retrovir. 17:487-497. [DOI] [PubMed] [Google Scholar]
- 12.Kellam, P., C. A. B. Boucher, J. M. G. H. Tijnagel, and B. A. Larder. 1994. Zidovudine treatment results in the selection of human immunodeficiency virus type 1 variant whose genotype confer increasing levels of drug resistance. J. Gen. Virol. 75:341-351. [DOI] [PubMed] [Google Scholar]
- 13.Koch, N., N. Yahi, P. Colson, J. Fantini, and C. Tamalet. 1999. Genetic polymorphism near HIV-1 reverse transcriptase resistance-associated codons is a major obstacle for the line probe assay as an alternative method to sequence analysis. J. Virol. Methods 80:25-31. [DOI] [PubMed] [Google Scholar]
- 14.Kuritzkes, D. R., D. Shugarts, M. Bakhtiari, D. Poticha, J. Johnson, M. Rubin, T. R. Gingeras, M. Kennedy, and J. J. Eron. 2000. Emergence of dual resistance to zidovudine and lamivudine in HIV-1-infected patients treated with zidovudine plus lamivudine as initial therapy. J. Acquir. Immune Defic. Syndr. 23:26-34. [DOI] [PubMed] [Google Scholar]
- 15.Kusagawa, S., H. Sato, K. Kato, K. Nohtomi, T. Shiino, C. Samrith, H. B. Leng, T. Phalla, M. B. Heng, and Y. Takebe. 1999. HIV type 1 env subtype E in Cambodia. AIDS Res. Hum. Retrovir. 15:91-94. [DOI] [PubMed] [Google Scholar]
- 16.Kusagawa, S., H. Sato, S. Watanabe, K. Nohtomi, K. Kato, T. Shino, M. Thwe, K. Y. Oo, S. Lwin, R. Mra, B. Kywe, S. Yamazaki, and Y. Takebe. 1998. Genetic and serologic characterization of HIV type 1 prevailing in Myanmar (Burma). AIDS Res. Hum. Retrovir. 10:1379-1385. [DOI] [PubMed] [Google Scholar]
- 17.Larder, B. A., and S. D. Kemp. 1989. Multiple mutations in HIV-1 reverse transcriptase confer high level resistance to zidovudine. Science 246:1155-1158. [DOI] [PubMed] [Google Scholar]
- 18.Little, S. J., S. Holte, J. P. Routy, E. S. Daar, M. Markowitz, A. C. Collier, R. A. Koup, B. Conway, E. Connick, M. S. Saag, A. Mwatha, L. Corey, P. H. Keiser, M. Kilby, K. Dawson, J. M. Whitcomb, N. S. Hellmann, and D. D. Richman. 2001. Antiretroviral resistance and response to initial therapy among recently HIV infected subjects in North America. Antivir. Ther. 6(Suppl. 1):21. [Google Scholar]
- 19.Merel, P., I. Pellegrin, I. Garrigue, A. Caumont, M. H. Schrive, V. Birac, P. Bonot, and H. Fleury. 2001. Comparison of capillary electrophoresis sequencing with the new CEQ 2000 DNA analysis system to conventional gel-based systems for HIV drug resistance analysis. J. Virol. Methods 98:9-16. [DOI] [PubMed] [Google Scholar]
- 20.Motomura, K., S. Kusagawa, K. Kato, K. Nohtomi, H. H. Lwin, K. M. Tun, M. Thwe, K. Y. Oo, S. Lwin, O. Kyaw, M. Zaw, Y. Nagai, and Y. Takebe. 2000. Emergence of new forms of human immunodeficiency virus type 1 intersubtype recombinants in central Myanmar. AIDS Res. Hum. Retrovir. 20:1831-1843. [DOI] [PubMed] [Google Scholar]
- 21.Resch, W., N. Parkin, E. L. Stuelke, T. Watkins, and R. Swanstrom. 2001. A multiple-site-specific heteroduplex tracking assay as a tool for the study of viral population dynamics. Proc. Natl. Acad. Sci. USA 98:176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rousseau, M. N., L. Vergne, B. Montes, M. Peeters, J. Reynes, E. Delaporte, and M. Segondy. 2001. Patterns of resistance mutations to antiretroviral drugs in extensively treated HIV-1-infected patients with failure of highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 26:36-43. [DOI] [PubMed] [Google Scholar]
- 23.Rusconi, S., S. La Seta Catamancio, F. Sheridan, and D. Parker. 2000. A genotypic analysis of patients receiving zidovudine with either lamivudine, didanosine or zalcitabine dual therapy using the LiPA point mutation assay to detect genotypic variation at codons 41, 69, 70, 74, 184, and 215. J. Clin. Virol. 19:135-142. [DOI] [PubMed] [Google Scholar]
- 24.Rust, S., H. Funke, and G. Assmann. 1993. Mutagenically separated PCR (MS-PCR): a high specific one step procedure for easy mutation detection. Nucleic Acids Res. 21:3623-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruxrungtham, K., and P. Phanuphak. 2001. Update on HIV/AIDS in Thailand. J. Med. Assoc. Thai. 84(Suppl. 1):S1-S17. [PubMed] [Google Scholar]
- 26.Sato, H., Y. Tomita, K. Ebisawa, A. Hachiya, K. Shibamura, T. Shiino, R. Yang, M. Tatsumi, K. Gushi, H. Umeyama, S. Oka, Y. Takebe, and Y. Nagai. 2001. Augmentation of human immunodeficiency virus type 1 subtype E (CRF01_AE) multiple-drug resistance by insertion of a foreign 11-amino-acid fragment into the reverse transcriptase. J. Virol. 75:5604-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Servais, J., C. Lambert, E. Fontaine, J. M. Plesseria, I. Robert, V. Arendt, T. Staub, F. Schneider, R. Hemmer, G. Burtonboy, and J. C. Schmit. 2001. Comparison of DNA sequencing and a line probe assay for detection of human immunodeficiency virus type 1 drug resistance mutations in patients failing highly active antiretroviral therapy. J. Clin. Microbiol. 39:454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaffer, N., R. Chuachoowong, P. A. Mock, C. Bhadrakom, W. Siriwasin, N. L. Young, T. Chotpitayasunondh, S. Chearskul, A. Roongpisuthipong, P. Chinayon, J. Karon, T. D. Mastro, and R. J. Simonds. 1999. Short course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand. Lancet 353:773-780. [DOI] [PubMed] [Google Scholar]
- 29.UNAIDS. 2001. AIDS epidemic update. World Health Organization, Geneva, Switzerland.
- 30.Ungsedhapand, C., E. D. Kroon, S. Suwanagool, K. Ruxrungtham, N. Yimsuan, A. Sonjai, S. Ubolyam, S. Buranapraditkun, S. Tiengrim, N. Pakker, C. Kunanusont, J. M. Lange, D. A. Cooper, and P. Phanuphak. 2001. A randomized, open-label, comparative trial of zidovudine plus lamivudine versus zidovudine plus lamivudine plus didanosine in antiretroviral-naive HIV-1-infected Thai patients. J. Acquir. Immune Defic. Syndr. 27:116-123. [DOI] [PubMed] [Google Scholar]
- 31.Van Laethem, K., K. Van Vaerenbergh, J.-C. Schmit, S. Sprecher, P. Hermans, V. De Vroey, R. Schuurman, T. Harrer, M. Witvrouw, E. Van Wijngaerden, L. Styver, M. Van Ranst, J. Desmyter, E. De Clercq, and A.-M. Vandamme. 1999. Phenotypic assays and sequencing are less sensitive than point mutation assays for detection of resistance in mixed HIV-1 genotype populations. J. Acquir. Immune Defic. Syndr. 22:107-118. [DOI] [PubMed] [Google Scholar]
- 32.Villahermosa, M. L., I. Beck, L. Perez-Alvarez, G. Contreras, L. M. Frenkel, S. Osmanov, E. V. de Parga, E. Delgado, N. Manjon, M. T. Cuevas, M. M. Thomson, L. Medrano, and R. Najera. 2001. Detection and quantification of multiple drug resistance mutations in HIV-1 reverse transcriptase by an oligonucleotide ligation assay. J. Hum. Virol. 4:238-248. [PubMed] [Google Scholar]
- 33.Zollner, B., H.-H. Feucht, L. Weitner, A. Adam, M. Schroter, P. Schafer, and R. Laufs. 2001. Application of HIV-1 genotypic-resistance testing prevents the evolution of further resistance mutations in heavily pretreated patients. J. Clin. Virol. 21:37-45. [DOI] [PubMed] [Google Scholar]