Abstract
Neisseria gonorrhoeae strains with reduced susceptibility to cefixime (MICs, 0.25 to 0.5 μg/ml) were isolated from male urethritis patients in Tokyo, Japan, in 2000 and 2001. The resistance to cephems including cefixime and penicillin was transferred to a susceptible recipient, N. gonorrhoeae ATCC 19424, by transformation of the penicillin-binding protein 2 gene (penA) that had been amplified by PCR from a strain with reduced susceptibility to cefixime (MIC, 0.5 μg/ml). The sequences of penA in the strains with reduced susceptibilities to cefixime were different from those of other susceptible isolates and did not correspond to the reported N. gonorrhoeae penA gene sequences. Some regions in the transpeptidase-encoding domain in this penA gene were similar to those in the penA genes of Neisseria perflava (N. sicca), Neisseria cinerea, Neisseria flavescens, and Neisseria meningitidis. These results showed that a mosaic-like structure in the penA gene conferred reductions in the levels of susceptibility of N. gonorrhoeae to cephems and penicillin in a manner similar to that found for N. meningitidis and Streptococcus pneumoniae.
Gonococcal infections have existed as sexually transmitted diseases since early times and have never been regarded as intractable diseases. In Japan, the numbers of gonococcal infections, including those resistant to antimicrobial therapy, have gradually increased since the mid-1990s (11).
Penicillins and tetracyclines are used for the treatment of gonococcal urethritis worldwide. After the emergence and worldwide spread of penicillin- and tetracycline-resistant Neisseria gonorrhoeae strains, fluoroquinolones were recommended as the primary therapy for uncomplicated gonorrhea in many countries (24). Fluoroquinolones have been used extensively for the treatment of gonococcal urethritis due to their high degrees of efficacy against the disease. Intense selective pressure resulting from the continual exposure of N. gonorrhoeae to fluoroquinolones resulted in the emergence of resistant strains with altered GyrA and ParC proteins (3, 6, 21, 22, 23). In recent years, expanded-spectrum oral cephems have been widely used instead of fluoroquinolones for the treatment of gonorrhea in Japan. However, the emergence and spread of gonococci resistant to oral cephems have been reported (1, 13).
N. gonorrhoeae has three penicillin-binding proteins (PBPs), denoted PBPs 1, 2, and 3. PBPs 1 and 2 of N. gonorrhoeae are the major targets of β-lactam antibiotics. PBP 2, encoded by the penA gene, has an approximately 10-fold higher affinity for penicillin than PBP 1 (7). In previous reports, insertion of the Asp-345A codon into the penA gene has been proved to make a major contribution to the reduction of the affinity of gonococcal PBP 2 to penicillin (5). Other reports showed that C-terminal amino acid residues of the penA transpeptidase domain were also altered in penicillin-resistant N. gonorrhoeae (8, 18, 19). Enhancement of the efflux pump by mutations in the mtrR and penB loci was reported to be due to β-lactam resistance (9, 10).
In 2000 we isolated gonococcal strains with reduced susceptibilities to penicillin and cephems including cefixime, which is recommended as therapy for gonococcal urethritis, during an investigation into the cause of clinical failure in patients with gonococcal urethritis treated with oral cephems. This study was conducted to investigate the susceptibilities to various antimicrobials of clinical isolates of N. gonorrhoeae recently isolated in Japan and to clarify the mechanism of reduced susceptibility to cefixime in N. gonorrhoeae.
MATERIALS AND METHODS
Bacteria and media.
The N. gonorrhoeae strains used in this study were clinical strains isolated from male urethritis patients at the School of Medicine, Jikei University, and related hospitals in 2000 (February to July) and 2001 (February to March). The specimens were directly streaked onto Thayer-Martin selective agar (Becton Dickinson, Cockeysville, Md.) in the hospitals. The plates were placed in a Bio-Bag environmental chamber (type C; Becton Dickinson) and immediately transported to the laboratory, where they were incubated at 35°C for 48 h in a 5% CO2 atmosphere. The organisms were identified by Gram staining and by oxidase and catalase tests. The identities of isolates cultured on Chocolate II agar (Becton Dickinson) were further confirmed with a Gonochek-II kit (EY Laboratories, San Mateo, Calif.). N. gonorrhoeae isolates were maintained at −80°C in modified skim milk (15) until antimicrobial susceptibility testing. The isolates were tested for β-lactamase production by a nitrocefin method. In the antibiotic susceptibility test, 53 and 24 strains isolated in 2000 and 2001, respectively, were used. The penA sequences of six of the clinical gonococcal isolates described above (strains NG-3, NG-12, NG-25, NG-46, NG-48, and NG-83) were used. The bacteria were grown at 37°C under a 5% CO2 atmosphere on brain heart infusion agar (Difco Laboratories, Detroit, Mich.) including 5% sheep defibrinated blood (Nippon Bio-Test Laboratories Inc., Tokyo, Japan) for 48 h.
Susceptibility testing and antimicrobials.
The MICs were determined by an agar dilution method according to the approved guidelines of the National Committee for Clinical Laboratory Standards (14). The following reference antimicrobials were used: penicillin G (Banyu Pharmaceutical Co., Ltd., Tokyo, Japan); piperacillin, tazobactam-piperacillin, and cefteram (Toyama Chemical Co., Ltd., Tokyo, Japan); ceftriaxone (Nippon Roche Co., Ltd., Tokyo, Japan); flomoxef (Shionogi Pharmaceutical Co., Ltd., Osaka, Japan); aztreonam (Eizai Co., Ltd., Tokyo, Japan); spectinomycin and minocycline (Sigma Chemical Co., St. Louis, Mo.); cefixime and cefdinir (Fujisawa Co., Ltd., Osaka, Japan); cefpodoxime (GlaxoSmithKline Japan, Tokyo, Japan); cefodizime (Kyorin Pharmaceutical Co., Ltd., Tokyo, Japan); and levofloxacin (Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan). Cefixime, cefdinir, cefpodoxime, and levofloxacin were extracted from commercially available capsules or tablets. The purities of these four agents were above 99.8%, as measured by high-performance liquid chromatography (HPLC).
Genetic transformation.
Genomic DNA was prepared from an N. gonorrhoeae strain with reduced susceptibility to cefixime (strain NG-3). The penA amplicon used for transformation was amplified by PCR as follows. Bacteria were suspended in 50 μl of distilled water, subjected to one freeze-thaw cycle, heated at 100°C for 3 min, and then centrifuged at 10,000 × g for 5 min. The full-length gene was amplified by PCR from the supernatant with oligonucleotides NGPA-F and NGPA-R (Table 1) and Ex Taq polymerase (Takara Shuzo, Kyoto, Japan). PCR was performed as follows: 5 min of denaturation at 94°C and 35 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 0.5 min, and extension at 72°C for 2 min, concluding with a final extension at 72°C for 5 min. Transformation for homologous recombination of the penA gene was done with the PCR amplicon and by coincubation under static conditions. Transformants were selected on plates containing cefixime at a concentration of 0.0313 μg/ml.
TABLE 1.
Oligonucleotides used in this study
| Oligo-nucleotide | Sequence |
|---|---|
| NGPA-F | 5′-TCGGGCAATACCTTTATGGTGGAACAT-3′ |
| NGPA-R | 5′-ACAACGGCGGCGGGGATATAACT-3′ |
| Fs1 | 5′-CAAAGATAGAAGCAGCCTGTGT-3′ |
| Fs2 | 5′-GATATTGACGGCAAAGGTCAGGAAGGT-3′ |
| Fs3 | 5′-CTTTGGATGTGCGCGGCATTATGCA-3′ |
| Rs1 | 5′-GCCGTCGGTATATTCGCCCTGA-3′ |
| Rs2 | 5′-CAGCCAAAGGGGTTAACTTGCTGAAC-3′ |
| Rs3 | 5′-TTCTCAACAAACCTGCAGTTTC-3′ |
| Rs4 | 5′-CTTTGCCGTTTTGCGGGGCTTTATTGC-3′ |
| Aat | 5′-CATCCAAAGAGGGGTAAACATGGGTGACG-3′ |
| r1 | 5′-GCGCAACCGTGCCGTAACCGATATGATCG-3′ |
Nucleotide sequence of N. gonorrhoeae penA gene.
The full-length penA gene was amplified by PCR with oligonucleotides NGPA-F and NGPA-R (Table 1). The amplicons were purified with a PCR product presequencing kit (Amersham Pharmacia Biotech, Tokyo, Japan). The cycling reaction was performed with Thermo Sequenase DNA polymerase (Amersham Pharmacia Biotech) and oligonucleotides Fs1, Fs2, Fs3, Rs1, Rs2, Rs3, and Rs4 (Table 1). Sequencing was carried out with a DSQ-1000 sequencer (Shimadzu, Kyoto, Japan). Primer Fs1-3 was used for sequencing of the forward sequence, and primer Rs1-4 was used for sequencing of the reverse sequence (Table 1).
Restriction fragment length polymorphism analysis of penA gene.
The amplicon obtained by PCR with primers Aat and r1 (Table 1) was digested with the restriction endonuclease AatII (New England Biolabs, Inc., Beverly, Mass.). Restriction digests were analyzed by electrophoresis on 4% agarose gels (Agarose X; Nippon Gene, Toyama, Japan). Primer Aat makes a site that is digested with AatII if the GAC codon Asp-345A is inserted in the penA gene.
Nucleotide sequence accession number.
The penA sequence of N. gonorrhoeae NG-3 has been deposited in the DDBJ data library under accession number AB071984.
RESULTS
Antimicrobial susceptibility and β-lactamase production.
The MICs of various antimicrobials and β-lactamase production were determined for 53 and 24 clinical isolates recovered in 2000 and 2001, respectively. The MICs at which 50% of isolates are inhibited (MIC50s) and the MIC90s of various antimicrobials for the clinical isolates are shown in Table 2. Nine of 53 strains (17.0%) isolated in 2000 and 4 of 24 strains (16.7%) isolated in 2001 showed reduced susceptibilities to cefixime (MICs, 0.25 and 0.5 μg/ml, respectively). These strains also exhibited reduced susceptibilities to penicillin and other β-lactams, and some of them were cross-resistant to fluoroquinolones, spectinomycin, and minocycline. There were no apparent differences in the MIC90s of any antimicrobials for the strains isolated in 2000 and 2001. However, the MIC50s of some β-lactams for the isolates recovered in 2001 were four- to eightfold higher than those for the isolates recovered in 2000. β-Lactamase production was not detected in any of the clinical isolates tested.
TABLE 2.
Susceptibilities of clinical isolates of N. gonorrhoeae from male urethritis patients in 2000 and 2001
| Antibiotic | MIC (μg/ml) |
MIC ratioa |
||||
|---|---|---|---|---|---|---|
| 2000 (n = 53) |
2001 (n = 24) |
50% | 90% | |||
| 50% | 90% | 50% | 90% | |||
| Penicillin G | 0.5 | 2 | 1 | 2 | 2 | 1 |
| Piperacillin | 0.0313 | 0.0625 | 0.0625 | 0.125 | 2 | 2 |
| Tazobactam-piperacillin | 0.0625 | 0.125 | 0.0625 | 0.125 | 1 | 1 |
| Cefixime | 0.008 | 0.25 | 0.0313 | 0.25 | 4 | 1 |
| Cefteram | 0.0156 | 0.5 | 0.0625 | 0.25 | 4 | 0.5 |
| Cefdinir | 0.0156 | 1 | 0.0313 | 1 | 2 | 1 |
| Cefpodoxime | 0.0156 | 1 | 0.125 | 1 | 8 | 1 |
| Ceftriaxone | 0.004 | 0.0625 | 0.0156 | 0.0313 | 4 | 0.5 |
| Cefodizime | 0.0156 | 0.0625 | 0.0313 | 0.0625 | 2 | 1 |
| Aztreonam | 0.125 | 4 | 0.5 | 4 | 4 | 1 |
| Flomoxef | 0.5 | 2 | 2 | 4 | 4 | 2 |
| Levofloxacin | 0.25 | 4 | 4 | >4 | 16 | NCb |
| Spectinomycin | 4 | 4 | NDc | ND | ||
| Minocycline | 0.25 | 0.5 | ND | ND | ||
MIC for 2001/MIC for 2000.
NC, not calculated.
ND, not determined.
Antimicrobial susceptibility of the transformant with the penA gene derived from an N. gonorrhoeae strain with reduced susceptibility to cefixime.
To investigate whether a reason for the reduced susceptibility to cefixime was alteration of PBP 2, the penA gene derived from strain NG-3, which had reduced susceptibility to cefixime, was transformed into N. gonorrhoeae ATCC 19424 (cefixime MIC, 0.001 μg/ml). After transformation of the penA gene, many transformants were obtained on plates containing 0.0313 μg of cefixime per ml. These transformants had similar susceptibility profiles. Table 3 shows the susceptibilities of the recipient (ATCC 19424) and one of the transformants (S1-05). The MICs of cefixime and ceftriaxone for the transformant were 0.0625 and 0.002 μg/ml, respectively. The susceptibilities of the transformant to penicillin G, cefixime, cefdinir, cefpodoxime, and aztreonam were reduced 64- to 128-fold, and those to piperacillin and ceftriaxone were reduced 2- to 8-fold. There were some discrepancies in antimicrobial susceptibilities between the transformant and a clinical isolate, NG-3, the donor of the resistance gene.
TABLE 3.
MICs of various antibiotics for N. gonorrhoeae ATCC 19424, transformant S1-05, and NG-3
| Antibiotic | MIC (μg/ml) |
MIC ratioa | ||
|---|---|---|---|---|
| ATCC 19424 | Transformant (S1-05) | NG-3 | ||
| Penicillin G | 0.004 | 0.25 | 2 | 64 |
| Piperacillin | 0.0005 | 0.001 | 0.0625 | 2 |
| Tazobactam-piperacillin | 0.0005 | 0.002 | 0.125 | 4 |
| Cefixime | 0.001 | 0.0625 | 0.5 | 64 |
| Cefteram | 0.004 | 0.125 | 0.5 | 32 |
| Cefdinir | 0.001 | 0.125 | 1 | 128 |
| Cefpodoxime | 0.001 | 0.125 | 2 | 128 |
| Ceftriaxone | 0.00025 | 0.002 | 0.0625 | 8 |
| Cefodizime | 0.00025 | 0.004 | 0.125 | 16 |
| Aztreonam | 0.008 | 1 | 8 | 128 |
| Flomoxef | 0.0313 | 0.5 | 2 | 16 |
| Levofloxacin | <0.004 | <0.004 | 8 | NCb |
| Spectinomycin | 2 | 1 | 4 | 0.5 |
MIC for transformant/MIC for ATCC 19424.
NC, not calculated.
Sequences of penA genes in strains with reduced susceptibilities to cefixime.
The full-length penA sequences were determined by using five strains (strains NG-3, NG-25, NG-46, and NG-48, isolated in 2000, and strain NG-83, isolated in 2001) with reduced susceptibilities to cefixime (MICs, 0.5 and 0.25 μg/ml for the strains isolated in 2000 and 2001, respectively) and one cefixime-susceptible strain (strain NG-12, isolated in 2000; cefixime MIC, 0.008 μg/ml). Figure 1 shows the full-length sequences of the penA genes of NG-3 (Fig. 1B) and NG-12 (Fig. 1C). In cefixime-susceptible strain NG-12, the penA gene sequence corresponded to that of penicillin-susceptible N. gonorrhoeae LM306 (GenBank accession no. M320921; Fig. 1A) except for an extra aspartate codon and an extra 2 bp. The penA gene of strain NG-3, which had reduced susceptibility to cefixime, did not have the extra codon (Fig. 1B), and the sequence was not consistent with the sequence reported in the database. Of 581 amino acids in the PBP 2 sequence of NG-3, 59 (10.2%) amino acids were different from the sequence of NG-12, in addition to 1 amino acid insertion and a defect.
FIG.1.
Nucleotide sequence of penA gene of N. gonorrhoeae. The sequences of the penA genes of penicillin-susceptible strain LM306 (GenBank accession no. M32091) (A), the strain with reduced susceptibility to cefixime (strain NG-3) (B), and cefixime-susceptible strain NG-12 (C) are shown. The insertion of an extra aspartate (Asp-345A) is shown in cefixime-susceptible strain NG-12 but is not shown in the strain with reduced susceptibility to cefixime, NG-3. Asp∗, Asp-345A. The Ser-X-X-Lys, Ser-X-Asn, and Lys-Thr-Gly conserved motifs are indicated by underlining.
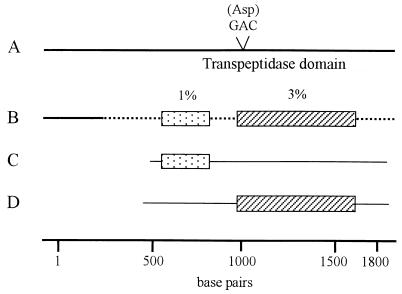
As a result of restriction fragment length polymorphism analysis, it was found that the penA genes of all strains for which cefixime MICs were below 0.125 μg/ml had an extra aspartate (GCA) codon (data not shown). The sequences of the penA genes of strains NG-25, NG-46, NG-48, and NG-83 (strains with reduced susceptibilities to cefixime) were the same as that of NG-3 except for a few mutations (data not shown). These penA genes had a mosaic-like structure that included regions that were quite similar to each region of the penA genes of Neisseria perflava (Neisseria sicca) and Neisseria cinerea (Fig. 2) as well as to those of Neisseria flavescens and Neisseria meningitidis (data not shown). This mosaic-like structure was mainly observed in the region of the transpeptidase-encoding domain of penA. The active-site serine residue (Ser-X-X-Lys) as well as the Ser-X-Asn and the Lys-Thr-Gly motifs were conserved in the penA sequence.
FIG. 2.
Schematic representation of mosaic-like penA genes of Neisseria strains. The penA gene of N. gonorrhoeae and the coding region for PBP 2 are represented in the diagram. The penA genes of cefixime-susceptible N. gonorrhoeae strain NG-12 (A), an N. gonorrhoeae strain with reduced susceptibility to cefixime (strain NG-3) (B), N. cinerea strain LPN3173 (C), and N. perflava (N. sicca) strain 1654/1659 (D) are shown. The nucleotide sequence divergences (in percent) between regions of the N. gonorrhoeae NG-3 penA genes and the corresponding regions in the penA genes of N. cinerea LPN3173 (░⃞) and N. perflava (N. sicca) 1654/1659 (▨) are shown.
DISCUSSION
In Japan, the emergence of resistance to cephems in N. gonorrhoeae is a serious concern. A more serious problem, however, is that these isolates are already resistant to non-β-lactam antimicrobials (1, 13). N. gonorrhoeae strains with reduced susceptibilities to cefixime from male urethritis patients at hospitals in Tokyo were also resistant to non-β-lactam antimicrobials, including fluoroquinolones. From the results of susceptibility testing with the strains isolated in 2000 and 2001, it was revealed that the numbers of strains with reduced susceptibilities to β-lactams, such as cefixime, cefteram, cefdinir, cefpodoxime, and aztreonam, had increased. Similar results were obtained with cefozopran-resistant N. gonorrhoeae strains isolated in Kitakyushu, Japan, for which the cefixime MICs were 0.125 to 0.5 μg/ml (13).
It has been reported that N. gonorrhoeae strains with reduced susceptibilities to cephems evolved by the acquisition of β-lactamases, target modification (alteration of PBPs), alteration of outer membrane transport, or enhancement of MtrCDE efflux pumps (10). β-Lactamase production did not contribute to the resistance in the strains tested in this study because β-lactamase activity was not detected in any of the strains. Transformation of the penA gene from a strain with reduced susceptibility to cefixime showed that the reduction in susceptibility to β-lactams was caused by PBP alterations. However, the reasons for the differences in the ratios of the MICs for the transformants to the MICs for the recipients between some β-lactams and the differences in susceptibilities between transformants and clinical isolates have not been identified. The latter reasons for these differences were considered enhancement of efflux pumps, alteration of outer membrane transport, and other PBP mutations.
In previous reports, insertion of the Asp-345A codon into the penA gene has proved to make a major contribution to the reduction of the affinity of gonococcal PBP 2 to penicillin (5). In this study, all strains for which cefixime MICs were below 0.125 μg/ml had an extra aspartate codon (Asp-345A) and showed reduced susceptibilities to penicillin, as reported previously (5). On the other hand, this extra codon was not detected in the strains for which cefixime MICs were 0.25 and 0.5 μg/ml.
The sequence of the penA gene of one strain, NG-3, with reduced susceptibility to cefixime (MIC, 0.5 μg/ml) was not completely consistent with the sequence reported in the database and had a mosaic-like structure that included a region whose sequence was quite similar to the sequences of the penA genes of N. perflava (N. sicca) and N. cinerea (Fig. 2) as well as those of N. flavescens and N. meningitidis (data not shown). Similar results have been reported from studies of the sequences of the penA genes of penicillin-resistant strains of N. meningitidis and Neisseria spp. (2, 4, 12, 16, 18, 20). One of the donors conferring the penA penicillin resistance gene to N. meningitidis has been identified as the naturally penicillin-resistant species N. flavescens (20). An N. gonorrhoeae penA gene with a mosaic-like structure that confers reduced susceptibility to cefixime might have been constructed by a medley of partial penA genes from N. perflava (N. sicca), N. cinerea, N. flavescens, and N. meningitidis. The reduction of susceptibility to cephems, including cefixime, in this study might have evolved by genetic exchange between commensal resistant Neisseria spp. and the original susceptible gonococci.
N. gonorrhoeae is one of the bacteria isolated from patients with sexually transmitted diseases. It has recently been reported that, in Japan, N. gonorrhoeae has been isolated from areas unrelated to the urethra, such as the pharynx (17). In the present study it was clear that the source of infection was oral sex for two of four patients from whom N. gonorrhoeae strains for which the cefixime MIC was 0.5 μg/ml were isolated. We speculate that a penA gene with a novel type of mosaic-like structure might have emerged by the transduction of regions from the penA genes of Neisseria spp. Due to the diversity of commercial sex, N. gonorrhoeae can inhabit the pharynx, and gene transformation between N. gonorrhoeae and other Neisseria spp. might proceed.
Our preliminary study with penA genes from isolates with reduced susceptibilities to cefixime (cefixime MICs, 0.0625 to 0.125 μg/ml) recovered in 2001 showed that the penA genes of these strains also had a mosaic-like structure and did not have the Asp-345A codon insert. This penA gene was different from that found in strain NG-3 in the present study (data not shown). The preliminary information presented above and the results obtained in this study suggest that the complicated process concerning the evolution of resistance in N. gonorrhoeae might be developing, and more attention should be paid to the emergence of resistance in Neisseria spp., including N. gonorrhoeae.
REFERENCES
- 1.Akasaka, S., T. Muratani, Y. Yamada, H. Inatomi, K. Takahashi, and T. Matsumoto. 2001. Emergence of cephem- and aztreonam-high-resistant Neisseria gonorrhoeae that does not produce beta-lactamase. J. Infect. Chemother. 7:49-50. [DOI] [PubMed] [Google Scholar]
- 2.Antignac, A., P. Kriz, G. Tzanakaki, J. M. Alonso, and M. K. Taha. 2001. Polymorphism of Neisseria meningitidis penA gene associated with reduced susceptibility to penicillin. J. Antimicrob. Chemother. 47:285-296. [DOI] [PubMed] [Google Scholar]
- 3.Belland, R. J., S. G. Morrison, C. A. Ison, and W. M. Huang. 1994. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone-resistant isolates. Mol. Microbiol. 14:371-380. [DOI] [PubMed] [Google Scholar]
- 4.Bowler, L. D., Q. Y. Zhang, J. Y. Riou, and B. G. Spratt. 1994. Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in N. meningitidis: natural events and laboratory simulation. J. Bacteriol. 176:333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brannigan, J. A., I. A. Tirodimos, Q. Y. Zhang, C. G. Dowson, and B. G. Spratt. 1990. Insertion of an extra amino acid is the main cause of the low affinity of penicillin-binding protein 2 in penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 4:913-919. [DOI] [PubMed] [Google Scholar]
- 6.Deguchi, T., M. Yasuda, M. Nakano, S. Ozeki, T. Ezaki, I. Saito, and Y. Kawada. 1996. Quinolone-resistant Neisseria gonorrhoeae: correlation of alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV with antimicrobial susceptibility profiles. Antimicrob. Agents Chemother. 40:1020-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dougherty, T. J., A. E. Koller, and A. Tomasz. 1980. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 18:730-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowson, C. G., A. E. Jephcott, K. R. Gough, and B. G. Spratt. 1989. Penicillin-binding protein 2 genes of non-beta-lactamase-producing, penicillin-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 3:35-41. [DOI] [PubMed] [Google Scholar]
- 9.Faruki, H., and P. F. Sparling. 1986. Genetics of resistance in a non-beta-lactamase-producing gonococcus with relatively high-level penicillin resistance. Antimicrob. Agents Chemother. 30:856-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill, M. J., S. Simjee, K. Al-Hattawi, B. D. Robertson, C. S. Easmon, and C. A. Ison. 1998. Gonococcal resistance to beta-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob. Agents Chemother. 42:2799-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumamoto, Y., T. Tsukamoto, I. Nishiya, H. Akaza, M. Noguchi, S. Kamidono, et al. 1999. Sexually transmitted disease surveillance in Japan (rate per 100,000/year by disease, age and gender: 1998). Jpn. J. Sex. Transm. 10:40-60. [Google Scholar]
- 12.Lujan, R., Q. Y. Zhang, J. A. Saez Nieto, D. M. Jones, and B. G. Spratt. 1991. Penicillin-resistant isolates of Neisseria lactamica produce altered forms of penicillin-binding protein 2 that arose by interspecies horizontal gene transfer. Antimicrob. Agents Chemother. 35:300-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muratani, T., S. Akasaka, T. Kobayashi, Y. Yamada, H. Inatomi, K. Takahashi, and T. Matsumoto. 2001. Outbreak of cefozopran (penicillin, oral cephems, and aztreonam)-resistant Neisseria gonorrhoeae in Japan. Antimicrob. Agents Chemother. 45:3603-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial susceptibility testing, 9th informational supplement M7-A4 (M100-S9). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Obara, Y., S. Yamai, T. Nikkawa, Y. Shimoda, and Y. Miyamoto. 1981. Preservation and transportation of bacteria by a simple gelatin disk method. J. Clin. Microbiol. 14:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saez-Nieto, J. A., R. Lujan, J. V. Martinez-Suarez, S. Berron, J. A. Vazquez, M. Vinas, and J. Campos. 1990. Neisseria lactamica and Neisseria polysaccharea as possible sources of meningococcal β-lactam resistance by genetic transformation. Antimicrob. Agents Chemother. 34:2269-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saika, T., T. Nishiyama, A. Kanayama, I. Kobayashi, H. Nakayama, M. Tanaka, and S. Naito. 2001. Comparison of Neisseria gonorrhoeae isolates from the genital tract and pharynx of two gonorrhea patients. J. Infect. Chemother. 7:175-179. [DOI] [PubMed] [Google Scholar]
- 18.Smith, J. M., C. G. Dowson, and B. G. Spratt. 1991. Localized sex in bacteria. Nature 349:29-31. [DOI] [PubMed] [Google Scholar]
- 19.Spratt, B. G. 1988. Hybrid penicillin-binding proteins in penicillin-resistant strains. Nature 332:173-176. [DOI] [PubMed] [Google Scholar]
- 20.Spratt, B. G., Q. Y. Zhang, D. M. Jones, A. Hutchison, J. A. Brannigan, and C. G. Dowson. 1989. Recruitment of a penicillin-binding protein gene from Neisseria flavescens during the emergence of penicillin resistance in Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 86:8988-8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su, X., and I. Lind. 2001. Molecular basis of high-level ciprofloxacin resistance in Neisseria gonorrhoeae strains isolated in Denmark from 1995 to 1998. Antimicrob. Agents Chemother. 45:117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka, M. H. Nakayama, M. Haraoka, T. Saika, I. Kobayashi, and S. Naito. 2000. Susceptibilities of Neisseria gonorrhoeae isolate containing amino acid substitutions in GyrA, with or without substitutions in ParC, to newer fluoroquinolones and other antibiotics. Antimicrob. Agents Chemother. 45:192-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka, M., S. Sakuma, K. Takahashi, T. Nagahuzi, T. Saika, I. Kobayashi, and J. Kumazawa. 1998. Analysis of quinolone resistance mechanisms in Neisseria gonorrhoeae isolates in vivo. Sex. Transm. Dis. 74:59-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. 1989. STD treatment strategies. Report WHO/VDT/89.447. World Health Organization, Geneva, Switzerland.