Abstract
Laboratory strains of Mycosphaerella graminicola with decreased susceptibilities to the azole antifungal agent cyproconazole showed a multidrug resistance phenotype by exhibiting cross-resistance to an unrelated chemical, cycloheximide or rhodamine 6G, or both. Decreased azole susceptibility was found to be associated with either decreased or increased levels of accumulation of cyproconazole. No specific relationship could be observed between azole susceptibility and the expression of ATP-binding cassette (ABC) transporter genes MgAtr1 to MgAtr5 and the sterol P450 14α-demethylase gene, CYP51. ABC transporter MgAtr1 was identified as a determinant in azole susceptibility since heterologous expression of the protein reduced the azole susceptibility of Saccharomyces cerevisiae and disruption of MgAtr1 in one specific M. graminicola laboratory strain with constitutive MgAtr1 overexpression restored the level of susceptibility to cyproconazole to wild-type levels. However, the level of accumulation in the mutant with an MgAtr1 disruption did not revert to the wild-type level. We propose that variations in azole susceptibility in laboratory strains of M. graminicola are mediated by multiple mechanisms.
In wheat-growing areas Septoria tritici blotch, caused by the fungus Mycosphaerella graminicola (anamorph, Septoria tritici), is recognized as a major disease. The resulting loss in yield is estimated to be millions of metric tons of grain and billions of U.S. dollars each year (11). Therefore, control of this disease either by resistance breeding or through chemical control is of major importance.
An important group of antifungal agents used to control M. graminicola is the sterol demethylation inhibitors (DMIs), which interfere with ergosterol biosynthesis through inhibition of the sterol P450 14α-demethylase (P45014DM). Azole antifungal agents in which the triazole ring acts as the active moiety are the most important group of DMIs. They have broad-spectrum antifungal activities, protective and curative properties, and low levels of phytotoxicity. Although mutants with resistance to DMIs could easily be obtained in the laboratory, the risk of resistance development in the field was initially considered low, as laboratory mutants generally suffered a fitness penalty (10). At present, resistance to azoles is widespread in various foliar pathogens, such as powdery mildews and scab (18). However, these pathogens can still be controlled by a limited number of DMIs with relatively high levels of intrinsic activity. This is also true for the control of septoria blotch of wheat, in which, despite the intensive use of cyproconazole and other azole antifungal agents, no indications of decreased susceptibilities to these compounds have been found (13).
Resistance to azole antifungal agents can be caused by alterations in sterol biosynthesis (20), mutations of the P45014DM target site (19, 32), or increased levels of expression of the P45014DM-encoding gene, CYP51 (15, 37). Another important resistance mechanism is reduction of the intracellular concentration of the antifungal agent by means of an increased active efflux system. This mechanism operates in a broad variety of both plant and animal pathogens and is attributed to the increased activities of ATP-binding cassette (ABC) transporters (2, 8, 9). ABC transporters became known for their role in multidrug resistance (MDR) in human tumor cells (21). They also function in MDR of filamentous fungi to antifungal agents and unrelated chemicals (1, 3, 33).
We are interested in the role of ABC transporters from M. graminicola in pathogenesis and in the susceptibility of this fungus to antifungal agents. To assess the roles of these transporters in the azole susceptibilities of M. graminicola, we selected from two genetically independent strains laboratory strains with decreased susceptibilities to the azole antifungal agent cyproconazole. All strains were analyzed for their susceptibilities to azoles and other chemically unrelated compounds, their levels of accumulation of [14C]cyproconazole, and their levels of expression of five ABC transporter genes (MgAtr1 to MgAtr5) and the sterol P450 14α-demethylase gene (CYP51). MgAtr1 was disrupted by Agrobacterium tumefaciens-mediated transformation in strains with constitutive MgAtr1 overexpression, and the transformants were phenotypically characterized. The results presented indicate that in M. graminicola multiple mechanisms may contribute to the variations in susceptibilities to azole antifungal agents.
MATERIALS AND METHODS
Fungal material and culture conditions.
In this study two field isolates of M. graminicola were used: isolate I323 was isolated in The Netherlands in 1981 (23), and isolate M1 was collected in France in 1993 and was provided by J. M. Seng (Biotransfer, Montreuil, France). Strains were grown yeast-like in liquid yeast-sucrose medium (yeast extract, 10 g/liter; sucrose, 10 g/liter) at 18°C and 140 rpm or on solid V8-agar plates (50% V8 vegetable juice, 50% water, 2.5% agar) at 18°C. The mycelium used in the accumulation studies was obtained by inoculating 100 ml of Czapek Dox-mycological peptone (Czapek Dox medium, 33.4 g/liter; mycological peptone, 5 g/liter) with 3 × 104 cells per ml and incubation on a rotary shaker (25°C, 140 rpm) for an additional 3 days.
Complementation of Saccharomyces cerevisiae was performed with strain AD12345678 (Δyor1 Δsnq2 Δpdr5 Δpdr10 Δpdr11 Δycf1 Δpdr3 Δpdr15 Δura3) (6).
Susceptibility assays and isolation of laboratory strains.
The antifungal agents tested were the triazoles cyproconazole (Syngenta, Basel, Switzerland), propiconazole (Syngenta), and tebuconazole (Bayer AG); the protein synthesis inhibitor cycloheximide (Sigma); and the dye rhodamine 6G (Sigma). The MICs for yeast-like growing cells were determined on V8-agar plates or potato dextrose agar plates (PDA; 39 g/liter). Toxicity tests were performed by spotting 5 μl of a suspension of cells (harvested from 3-day-old liquid medium with a density of 4 × 105 cells per ml) on 9-ml petri dishes containing V8-agar or PDA amended with different concentrations of toxicants. The concentrations of the compounds used in the susceptibility assays ranged from 0.025 to 1.5 mg/liter for the triazoles, from 2.5 to 1,500 mg/liter for cycloheximide, and from 2.5 to 250 mg/liter for rhodamine 6G. Experiments were performed three times in triplicate, and MICs were assessed visually after 10 days of incubation at 18°C in the dark.
M. graminicola strains with decreased susceptibilities to the azole antifungal agent cyproconazole were isolated by plating 105 yeast-like cells on V8-agar in 14-cm petri dishes amended with cyproconazole at three times the MIC for the wild-type parent strains. After 10 days of incubation colonies were isolated from these plates.
Assays of the susceptibilities of S. cerevisiae were performed on solid synthetic medium containing Bacto Yeast Nitrogen Base without amino acids (6.7 g/liter), dropout mix (2 g/liter containing amino acids without uracil), galactose (20 g/liter), Noble agar (20 g/liter), and cycloheximide or cyproconazole at various concentrations. Cycloheximide was used at 0.01, 0.05, 0.1, and 0.25 μg/ml; cyproconazole was used at 0.003, 0.01, and 0.025 μg/ml; and rhodamine 6G was used at 0.5, 1, and 5 μg/ml. Cultures of S. cerevisiae were grown overnight in liquid synthetic medium at 30°C. The overnight culture was diluted to an optical density at 600 nm of 0.5, and subsequently, 5 or 10 μl was spotted onto the plates. Drug susceptibility was scored visually after incubation for 3 days at 30°C in the dark.
Gene disruption.
A. tumefaciens-mediated transformation was used to disrupt MgAtr1 in laboratory-generated strains I323C1 and M1C4. Generation of disruption constructs and selection of transformants with the disrupted MgAtr1 gene were performed as described previously (42).
Accumulation of [14C]cyproconazole.
Mycelium was homogenized and harvested by filtering over 0.85-mm- and 55-μm-pore-size filters. Subsequently, mycelium was washed with 50 mM sodium phosphate buffer (pH 6.0), resuspended in 50 mM sodium phosphate buffer (pH 6.0; 1% glucose) at a density of 6 mg (wet weight) per ml, and incubated for 30 min at 25°C and 140 rpm. Subsequently, [14C]cyproconazole (kindly provided by Syngenta) was added to an external concentration of 100 μM. Mycelium (5 ml) was harvested at intervals of 10 min by vacuum filtration and washed five times with 5 ml of phosphate buffer, and the radioactivity in the biomass was measured with a Beckman LS6000TA liquid scintillation counter. The level of accumulation of [14C]cyproconazole was calculated as the number of nanomoles per milligram (dry weight). The energy dependency of [14C]cyproconazole accumulation was tested by the addition of carbonyl cyanide 3-chlorophenylhydrazone (CCCP; 20 μM) and subsequent measurement of the level of [14C]cyproconazole accumulation.
DNA and RNA manipulations.
M. graminicola genomic DNA isolated from 5-day-old yeast-like cells (29) was used to amplify the open reading frame of the M. graminicola CYP51 gene (GenBank accession no. AF263470). Amplification reaction volumes (50 μl) contained dATP, dCTP, dGTP, and dTTP (200 μM); primers (1.2 μM); AmpliTaq DNA polymerase (0.5 U; Perkin-Elmer); and a reaction buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 3 mM MgCl2). DNA was denatured for 3 min at 94°C, followed by 2 min at 50°C and 2 min at 72°C. This initial cycle was followed by 29 cycles of 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C. The amplification was stopped with an extension of 10 min at 72°C. The PCR primers used were CYP5′ (GGTACCATGGGTCTCCTCCAGGAAG) and CYP3′ (TCCCTCCTCTCCCACTTTAC). The amplification products were isolated from the agarose gel and directly sequenced.
Northern blot analysis was performed with RNA isolated from wild-type and laboratory-generated strains. The levels of expression of ABC transporter-encoding genes MgAtr1 (GenBank accession no. AJ243112), MgAtr2 (GenBank accession no. AJ243113) (41), MgAtr3 (GenBank accession no. AF364105), MgAtr4 (GenBank accession no. AF329852), and MgAtr5 (GenBank accession no. AF364104) (36) and the CYP51 gene were examined. Total RNA was isolated by using the TRIzol reagent (Life Technologies). RNA (10 μg) was separated on a 1.2% agarose gel containing glyoxal and transferred to Hybond N nylon membranes (Amersham). Equal loading and transfer of RNA were determined by staining Northern blots with methylene blue and hybridization with the 18S rRNA subunit of Aspergillus niger (26). Hybridizations were performed at 65°C in Nasmyth's hybridization solution (1.1 M NaCl, 0.3 M Na2HPO4, 0.011 M disodium EDTA, 1.85% sarcosyl, 18.5% dextran sulfate [pH 6.2], 100 μg of denatured herring sperm DNA per ml).
Complementation of S. cerevisiae.
Full-length cDNA clones of MgAtr1 were made from poly(A)+ RNA isolated from yeast-like cells of M. graminicola. Amplification of full-length cDNA was performed with the Advantage Klentaq polymerase mix (Clontech) according to the instructions of the manufacturer. cDNA clones were cloned in the yeast expression vector pYes2 (Invitrogen) and transformed into S. cerevisiae strain AD12345678. Yeast transformants containing the empty vector pYes2 were used as controls.
RESULTS
Assays of susceptibilities to azole antifungal agents.
The MICs of cyproconazole for field strains I323 and M1 were approximately 0.1 μg/ml on PDA and 0.3 μg/ml on V8-agar. Both strains showed similar cross-susceptibilities to the triazoles propiconazole and tebuconazole (data not shown).
To elucidate the potential role of ABC transporters in susceptibility to azoles, strains I323 and M1 were subjected to selection with cyproconazole. Strains with decreased susceptibilities to cyproconazole were isolated from V8-agar plates amended with cyproconazole at three times the MICs for both parent strains. For both I323 and M1 the frequency of resistant colonies was about 10−4. The relative decreases in the susceptibilities of several strains were determined and ranged between factors of 3 and 6 (Table 1). All strains showed cross-resistance to propiconazole and tebuconazole (data not shown). Repetitive subculturing of the strains under nonselective conditions showed that all I323-derived strains were stable, whereas M1-derived strains M1A1 and M1D1 lost their decreased susceptibilities to cyproconazole. The susceptibilities of the strains to several chemically unrelated chemicals were determined in order to study whether the strains had an MDR phenotype. Indeed, all strains exhibited a low degree of cross-resistance to cycloheximide and/or rhodamine 6G (Table 1).
TABLE 1.
Susceptibilities of field isolates, laboratory-generated strains with decreased sensitivities to azoles, and MgAtr1 mutants of M. graminicola with disrupted MgAtr1 to cyproconazole, cycloheximide, and rhodamine 6G
| Isolatea | MIC (μg/ml)
|
||
|---|---|---|---|
| Cyproconazole | Cycloheximide | Rhodamine 6G | |
| I323 | 0.1 | 500 | 25 |
| I323A1 | 0.4 | 1,000 | 25 |
| I323C1 | 0.75 | 1,500 | 150 |
| I323C4 | 0.5 | 1,000 | 25 |
| I323E1 | 0.75 | 1,500 | 50 |
| I323C1Δ1-1 | 0.1 | 25 | 25 |
| I323C1Δ1-2 | 0.1 | 25 | 25 |
| M1 | 0.1 | 500 | 15 |
| M1A1 | 0.4b | NDc | 30 |
| M1B1 | 0.3 | ND | 30 |
| M1C1 | 0.3 | ND | 30 |
| M1C4 | 0.5 | 1,500 | 100 |
| M1D1 | 0.3b | ND | 30 |
| M1C4Δ1-1 | 0.5 | 1,500 | 100 |
| M1C4Δ1-2 | 0.5 | 1,500 | 100 |
Strains I323A1, I323C1, I323C4, and I323E1 and strains M1A1, M1B1, M1C1, M1C4, and M1D1 are laboratory-generated strains derived from wild-type field isolates I323 and M1, respectively. Strains I323C1Δ1-1 and I323C1Δ1-2 and strains M1C4Δ1-1 and M1C4Δ1-2 are transformants of I323C1 and M1C4, respectively, with a disrupted allele of MgAtr1.
The strains were initially resistant; resistance was lost upon subculturing.
ND, not determined.
Accumulation of [14C]cyproconazole.
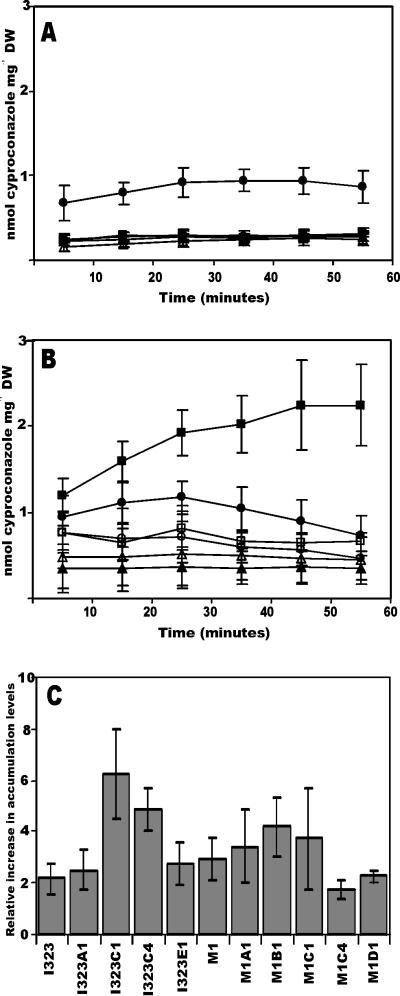
The decreased susceptibilities of the strains to cyproconazole could be due to reduced levels of intracellular accumulation of the antifungal agent in mycelial cells. Therefore, the levels of cyproconazole that accumulated in cells were measured over time (Fig. 1A and B). The levels of [14C]cyproconazole accumulation for the two wild-type strains, strains I323 and M1, did not differ significantly and amounted, on average, to 1 and 0.9 nmol of cyproconazole per mg (dry weight), respectively. In all strains derived from strain I323 the levels of accumulation of [14C]cyproconazole decreased significantly by factors of 3 to 4 (Fig. 1A). The levels of accumulation by M1-derived strains M1B1 and M1C1 decreased by factors of 2 to 3. In contrast, the level of accumulation of [14C]cyproconazole by strain M1C4 was higher than the level of accumulation by the wild type and increased over time (Fig. 1B). In all strains tested, the level of accumulation appeared to be due to an energy-dependent efflux, as the addition of CCCP increased the level of accumulation of cyproconazole (Fig. 1C).
FIG. 1.
(A) Levels of accumulation of [14C]cyproconazole in M. graminicola wild-type isolate I323 (•) and laboratory-generated strains with decreased cyproconazole susceptibilities, strains I323A1 (○), I323C1 (▴), I323C4 (▵), and I323E1 (▪). (B) Levels of accumulation of [14C]cyproconazole in wild-type isolate M1 (•) and laboratory-generated strains M1A1 (○), M1B1 (▴), M1C1 (▵), M1C4 (▪), and M1D1 (□). (C) Relative increases in levels of accumulation of [14C]cyproconazole measured 30 min after addition of CCCP. DW, dry weight.
Northern analysis.
The MDR phenotypes of the laboratory-generated strains could indicate that ABC transporters are involved in the mechanism of resistance (25). Therefore, the levels of expression of ABC transporter-encoding genes MgAtr1 to MgAtr5 were studied. Northern analysis with untreated yeast-like cells demonstrated that almost all laboratory-generated strains exhibited changes in the basal level of expression of at least one of the ABC transporter genes whose levels of expression were tested (Fig. 2). For instance, the levels of expression of MgAtr1 were highly increased in strains I323C1 and M1C4 compared to those in their respective wild-type parent strains. In addition, both strains showed increased levels of expression of MgAtr5. Even strain M1A1, which lost its resistance to cyproconazole, still showed a clear overexpression of an ABC transporter gene, e.g., MgAtr2. However, all these expression data indicate that there is no consistent correlation between the observed susceptibility profile of a strain and the profile of expression of any of the ABC transporter genes tested. All strains were also tested for levels of expression of the CYP51 gene. Only I323E1 showed, besides upregulation of MgAtr4, an increased level of CYP51 expression.
FIG. 2.
Expression of MgAtr1, MgAtr2, MgAtr4, MgAtr5, and CYP51 in M. graminicola wild-type strains (strains I323 and M1) and laboratory-generated strains (strains I323A1, I323C1, I323C4, I323E1, M1A1, M1B1, M1C1, M1C4, and M1D1). The 18S rRNA gene was used as a loading control.
Analysis of CYP51.
Using primers directed against the CYP51 gene from M. graminicola, a 1,903-bp fragment comprising the entire open reading frame was amplified from wild-type strains I323 and M1 and from laboratory-generated strains I323C1 and M1C4. The deduced amino acid sequences of the proteins encoded by the genes from the wild-type strains and both laboratory-generated strains were identical (data not shown).
Analysis of MgAtr1.
Strains I323C1 and M1C4 both constitutively overexpressed MgAtr1 but showed opposite accumulation patterns. The role of MgAtr1 in the decreased susceptibility to cyproconazole was analyzed in more detail by complementation of an S. cerevisiae mutant with MgAtr1 and by disruption of MgAtr1 in I323C1 and M1C4 by means of A. tumefaciens-mediated transformation.
The heterologous expression of MgAtr1 in S. cerevisiae showed that the presence of MgAtr1 results in the decreased susceptibilities of the yeast to both cycloheximide and cyproconazole (Fig. 3) Therefore, both cycloheximide and cyproconazole are potential substrates of MgAtr1.
FIG. 3.
Effect of heterologous expression of MgAtr1 from M. graminicola in S. cerevisiae AD12345678 on susceptibilities to cycloheximide, cyproconazole, and rhodamine 6G. Five or 10 μl (rows 1 and 2, respectively) of overnight cultures of two independent S. cerevisiae transformants containing the full-length cDNA clone of MgAtr1 (A) or control plasmid pYes2 (B) was diluted to an optical density at 600 nm of 0.5 and spotted. The concentrations of compounds in the agar are indicated in micrograms per milliliter.
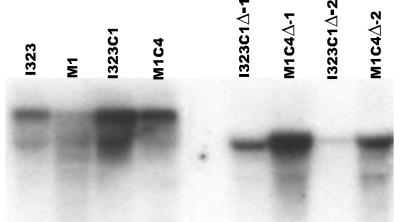
After A. tumefaciens-mediated transformation of both strain I323C1 and strain M1C4, two disruptants each containing a single copy of the transforming DNA were isolated and characterized with respect to MgAtr1 expression, their azole susceptibilities, and levels of cyproconazole accumulation. Northern analysis demonstrated that disruption of MgAtr1 resulted in the disappearance of full-length MgAtr1 mRNA (Fig. 4). The levels of accumulation of [14C]cyproconazole by the MgAtr1 disruptants of both I323C1 and M1C4 compared to those for their respective parent strains were similar (data not shown). The MgAtr1 disruptants derived from I323C1 had wild-type (strain I323) susceptibilities to cyproconazole and rhodamine 6G. Moreover, they became hypersensitive to cycloheximide (Fig. 5A), demonstrating a role for MgAtr1 in protection against these compounds in I323C1. In contrast, the MgAtr1 disruptants derived from M1C4 remained less susceptible to these compounds (Fig. 5B).
FIG. 4.
Expression of MgAtr1 in M. graminicola wild-type strains (strains I323 and M1), laboratory-generated strains (strains I323C1 and M1C4), and independent transformants of I323C1 and M1C4 with a disrupted MgAtr1 allele (strains I323C1Δ-1, I323C1Δ-2, M1C4Δ-1 and M1C4Δ-2)
FIG. 5.
Susceptibilities of M. graminicola wild-type strains, laboratory-generated azole-resistant strains, and MgAtr1 knockout mutants of laboratory-generated azole-resistant strains to cyproconazole, cycloheximide, and rhodamine 6G. (A) Wild-type isolate I323 (quadrant 1), laboratory-generated strain I323C1 (quadrant 2), and two independent transformants of I323C1 with the disrupted MgAtr1 allele (quadrants 3 and 4); (B) wild-type isolate M1 (quadrant 1), laboratory-generated strain M1C4 (quadrant 2), and two independent transformants of M1C4 with the disrupted MgAtr1 allele (quadrants 3 and 4). The concentrations of cyproconazole (I), cycloheximide (II), and rhodamine 6G (III) used are indicated in micrograms per milliliter.
DISCUSSION
Laboratory-generated strains of M. graminicola with decreased susceptibilities to the azole antifungal agent cyproconazole were selected at a rate of about 10−4. This frequency is not unique for M. graminicola, since similar frequencies have been observed for other filamentous fungi, e.g., Aspergillus nidulans and Nectria haematococca. Genetic analyses of azole-resistant strains of these organisms identified a polygenic system for azole resistance (22, 38). Recently, in Candida glabrata the development of so-called high-frequency azole resistance, which occurred at frequencies (2 × 10−4 to 4 × 10−4) comparable to those in M. graminicola, was described (31). We propose that the decreased susceptibilities of the laboratory-generated strains can be regarded as microbial resistance (34)
The susceptibilities of the laboratory-generated strains are still within the range observed in field populations of M. graminicola (13). This suggests that the mechanisms underlying the microbial resistance to azoles in laboratory strains can also occur in natural populations of the pathogen and, thus, contribute to the natural variation in baseline susceptibility.
Most strains of M. graminicola with decreased azole susceptibilities exhibited cross-resistance to the chemically unrelated compounds cycloheximide and rhodamine 6G. Such an MDR phenotype in strains with decreased azole susceptibilities has been described for the yeasts Candida albicans (33), C. glabrata (31), and S. cerevisiae (4) and the filamentous fungi A. nidulans (7), Botrytis cinerea (17), Penicillium digitatum (27), and Penicillium italicum (14). Most laboratory strains of M. graminicola with an MDR phenotype exhibited decreased levels of accumulation of cyproconazole. This observation suggests that the MDR is associated with an energy-dependent transport of drugs that results in a decreased cellular content of toxicants (8, 9). However, the level of accumulation of [14C]cyproconazole by strain M1C4 was higher than the level of accumulation by the wild-type strain and increased over time. This indicates that multiple mechanisms contribute to the variation in azole susceptibility in M. graminicola. The increased level of accumulation by M1C4 may be caused by cell wall changes that lead to an increased level of nonspecific binding of the compound to cell wall components. The relatively small increase in the level of accumulation of [14C]cyproconazole in M1C4 after addition of the uncoupler CCCP might offer support for this mechanism. Increased levels of accumulation of azoles have also been described for triadimenol-resistant laboratory strains of Ustilago maydis (40). An alternative mechanism for the increased level of accumulation of cyproconazole by strain M1C4 might be active sequestration of the fungicide in vacuoles. This protection mechanism is well described in plants, in which sequestration of endotoxins, heavy metals, and natural pigments occurs through a specific subclass of ABC transporters (30). In line with this explanation is the increase in the level of [14C]cyproconazole accumulation in M1C4 over time.
Differences in levels of drug accumulation can be mediated by changes in ABC transporter activity due to overexpression of ABC transporter genes. Therefore, the levels of expression of all ABC transporter genes cloned so far from M. graminicola (MgAtr1 to MgAtr5) were analyzed in all strains. Northern analysis indicated that the moderate changes in susceptibility to azoles are associated with profound changes in the levels of expression of the ABC transporter genes, suggesting that the regulation of the genes examined in the laboratory-generated strains is quite different from that in the parent strains. However, it is not possible to associate the level of expression of a specific ABC transporter gene with the observed phenotype. This indicates that multiple transporters may be involved in azole susceptibility or that the transporter of prime importance for azole transport in M. graminicola has not yet been identified.
We have studied the ABC transporter gene MgAtr1 in more detail since this gene was overexpressed in strains I323C1 and M1C4, which displayed decreased and increased levels of accumulation of cyproconazole, respectively. Complementation of an S. cerevisiae mutant with MgAtr1 decreases the susceptibilities of the yeast transformants to cyproconazole and cycloheximide. Disruption of MgAtr1 in I323C1 restored the susceptibilities to cyproconazole and rhodamine 6G to wild-type levels and even resulted in hypersusceptibility to cycloheximide. Therefore, all three compounds are potential substrates of MgAtr1 in M. graminicola.
Disruption of MgAtr1 in I323C1 did not cause the level of accumulation of cyproconazole to revert to the level found for the wild-type strain. Thus, our data prove that MgAtr1 can provide protection against azole antifungals in S. cerevisiae and M. graminicola, but they also show that the overall level of accumulation is not affected by the disruption of MgAtr1 in strain I323C1. These seemingly conflicting results are hard to explain but suggest that in I323C1 decreased influx and not increased efflux by MgAtr1 causes the reduced level of accumulation. Apparently, MgAtr1 can act as a determinant of azole susceptibility only when it is overexpressed and when azole influx is impaired. These results also imply that the overall level of accumulation of cyproconazole by laboratory strains of M. graminicola is probably not an indicator of either azole susceptibility or the exclusive involvement of ABC transporters. This contrasts with the situation in A. nidulans and B. cinerea (2, 35, 39) and emphasizes the complexity of the mechanisms that contribute to azole accumulation in M. graminicola.
As MgAtr1 was not upregulated in all laboratory strains selected and as disruption of MgAtr1 in M1C4 did not alter the phenotype, it is clear that besides overproduction of MgAtr1 other mechanisms act as determinants involved in the MDR phenotype. Disruption of other ABC transporter genes in the laboratory strains should indicate if additional ABC transporters are involved. This is well possible, since ABC transporters are members of a large protein superfamily and are known to possess overlapping substrate specificities (5, 25).
Sequencing of CYP51 from M. graminicola strains I323C1 and M1C4 did not show any of the point mutations reported to confer resistance to azole antifungal agents in C. albicans (12, 32). This indicates that, at least in these strains, mutations in the CYP51 gene are not involved in decreased susceptibility to cyproconazole. However, overexpression of CYP51, as observed in strain I323E1, could contribute to decreased susceptibility (15, 37).
In summary the data described here suggest that in laboratory strains of M. graminicola, multiple mechanisms contribute to the variation in azole susceptibility. One mechanism involves the upregulation of MgAtr1 and possibly more ABC transporter genes, which lead to increased levels of efflux of antifungal agents. Other mechanisms may involve changes in cell wall composition or sequestration of the antifungal agent in cellular compartments, resulting in increased levels of accumulation. Finally, reduced levels of passive influx may play a role. Multiple mechanisms probably operate in individual strains. Multiple mechanisms of azole resistance also function in C. albicans and P. digitatum (16, 24, 28). This situation complicates investigations on mechanisms of resistance to azoles in M. graminicola. At present, we are examining whether multiple mechanisms also account for the variations in azole susceptibility in field populations of M. graminicola.
Acknowledgments
We acknowledge G. H. J. Kema and C. Waalwijk for discussions within the Wageningen Mycosphaerella Group and P. J. G. M De Wit and D. Sanglard (University Hospital Lausanne, Lausanne, Switzerland) for critical reading of the manuscript. M. Collina and J. Zilverentant are acknowledged for technical assistance.
L.-H. Zwiers was financially supported by Syngenta, and I. Stergiopoulos was financially supported by the Training and Mobility of Researchers Programme-Marie Curie Research Grants, The European Commission (contract ERBFMBICT983558).
REFERENCES
- 1.Andrade, A. C., G. Del Sorbo, J. G. M. van Nistelrooy, and M. A. De Waard. 2000. The ABC transporter AtrB from Aspergillus nidulans mediates resistance to all major classes of fungicides and some natural toxic compounds. Microbiology (United Kingdom) 146:1987-1997. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, A. C., J. G. M. van Nistelrooy, R. B. Peery, P. L. Skatrud, and M. A. De Waard. 2000. The role of ABC transporters from Aspergillus nidulans in protection against cytotoxic agents and in antibiotic production. Mol. Gen. Genet. 263:966-977. [DOI] [PubMed] [Google Scholar]
- 3.Balzi, E., and A. Goffeau. 1995. Yeast multidrug resistance: the PDR network. J. Bioenerg. Biomembr. 27:71-76. [DOI] [PubMed] [Google Scholar]
- 4.Balzi, E., M. Wang, S. Leterme, L. VanDyck, and A. Goffeau. 1994. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator PDR1. J. Biol. Chem. 269:2206-2214. [PubMed] [Google Scholar]
- 5.Decottignies, A., and A. Goffeau. 1997. Complete inventory of the yeast ABC proteins. Nat. Genet. 15:137-145. [DOI] [PubMed] [Google Scholar]
- 6.Decottignies, A., A. M. Grant, J. W. Nichols, H. De Wet, D. B. McIntosh, and A. Goffeau. 1998. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem. 273:12612-12622. [DOI] [PubMed] [Google Scholar]
- 7.Del Sorbo, G., A. C. Andrade, J. G. Van Nistelrooy, J. A. Van Kan, E. Balzi, and M. A. De Waard. 1997. Multidrug resistance in Aspergillus nidulans involves novel ATP-binding cassette transporters. Mol. Gen. Genet. 254:417-426. [DOI] [PubMed] [Google Scholar]
- 8.Del Sorbo, G., H. Schoonbeek, and M. A. De Waard. 2000. Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fungal Genet. Biol. 30:1-15. [DOI] [PubMed] [Google Scholar]
- 9.De Waard, M. A. 1997. Significance of ABC transporters in fungicide sensitivity and resistance. Pestic. Sci. 51:271-275. [Google Scholar]
- 10.De Waard, M. A., and J. G. M. van Nistelrooy. 1990. Stepwise development of laboratory resistance to DMI-fungicides in Penicillium italicum. Neth. J. Plant Pathol. 96:321-329. [Google Scholar]
- 11.Eyal, Z., A. L. Scharen, J. M. Prescott, and M. van Ginkel. 1987. The septoria diseases of wheat: concepts and methods of disease managment. International Maize and Wheat Improvement Center, Mexico City, Mexico.
- 12.Gisi, U., K. M. Chin, G. Knapova, R. K. Farber, U. Mohr, S. Parisi, H. Sierotzki, and U. Steinfeld. 2000. Recent developments in elucidating modes of resistance to phenylamide, DMI and strobilurin fungicides. Crop Prot. 19:863-872. [Google Scholar]
- 13.Gisi, U., D. Hermann, L. Ohl, and C. Steden. 1997. Sensitivity profiles of Mycosphaerella graminicola and Phytophthora infestans populations to different classes of fungicides. Pestic. Sci. 51:290-298. [Google Scholar]
- 14.Guan, J., J. C. Kapteyn, A. Kerkenaar, and M. A. De Waard. 1992. Characterisation of energy-dependent efflux of imazalil and fenarimol in isolates of Penicillium italicum with a low, medium and high degree of resistance to DMI-fungicides. Neth. J. Plant Pathol. 98:313-324. [Google Scholar]
- 15.Hamamoto, H., K. Hasegawa, R. Nakaune, Y. J. Lee, Y. Makizumi, K. Akutsu, and T. Hibi. 2000. Tandem repeat of a transcriptional enhancer upstream of the sterol 14α-demethylase gene (CYP51) in Penicillium digitatum. Appl. Environ. Microbiol. 66:3421-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamamoto, H., O. Nawata, K. Hasegawa, R. Nakaune, Y. J. Lee, K. Makizumi, and T. Hibi. 2001. The role of the ABC transporter gene PMR1 in demethylation inhibitor resistance in Penicillium digitatum. Pestic. Biochem. Physiol. 70:19-26. [Google Scholar]
- 17.Hayashi, K., H. Schoonbeek, H. Sugiura, and M. A. De Waard. 2001. Multidrug resistance in Botrytis cinerea associated with decreased accumulation of the azole fungicide oxpoconazole and increased transcription of the ABC transporter gene BcatrD. Pestic. Biochem. Physiol. 70:168-179. [Google Scholar]
- 18.Hollomon, D. W. 1993. Resistance to azole fungicides in the field. Biochem. Soc. Trans. 21:1047-1051. [DOI] [PubMed] [Google Scholar]
- 19.Josep-Horne, T., D. Hollomon, R. S. T. Loeffler, and S. L. Kelly. 1995. Altered P450 activity associated with direct selection for fungal azole resistance. FEBS Lett. 374:174-178. [DOI] [PubMed] [Google Scholar]
- 20.Josep-Horne, T., N. J. Manning, D. Hollomon, and S. L. Kelly. 1995. Defective sterol Δ5(6)desaturase as a cause of azole resistance in Ustilago maydis. FEMS Microbiol. Lett. 374:1-2. [DOI] [PubMed] [Google Scholar]
- 21.Juliano, R. L., and V. Ling. 1976. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 455:152-162. [DOI] [PubMed] [Google Scholar]
- 22.Kalamarakis, A. E., M. A. De Waard, B. N. Ziogas, and S. G. Georgopoulos. 1991. Resistance to fenarimol in Nectria haematococca var. cucurbitae. Pestic. Biochem. Physiol. 40:212-220. [Google Scholar]
- 23.Kema, G. H. J., and C. H. Van Silfhout. 1997. Genetic variation for virulence and resistance in the wheat-Mycosphaerella graminicola pathosystem. III. Comparative seedling and adult plant experiments. Phytopathology 87:266-272. [DOI] [PubMed] [Google Scholar]
- 24.Kohli, A., N. F. N. Smriti, K. Mukhopadhyay, A. Rattan, and R. Prasad. 2002. In vitro low-level resistance to azoles in Candida albicans is associated with changes in membrane lipid fluidity and asymmetry. Antimicrob. Agents Chemother. 46:1046-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolaczkowski, M., A. Kolaczkowska, J. Luczynski, S. Witek, and A. Goffeau. 1998. In vivo characterization of the drug resistance profile of the major ABC transporters and other components of the yeast pleiotropic drug resistance network. Microb. Drug Resist. 4:143-158. [DOI] [PubMed] [Google Scholar]
- 26.Melchers, W. J., P. E. Verweij, P. Van den Hurk, A. Van Belkum, B. E. De Pauw, J. A. Hoogkamp Korstanje, and J. F. Meis. 1994. General primer-mediated PCR for detection of Aspergillus species. J. Clin. Microbiol. 32:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakaune, R., K. Adachi, O. Nawata, M. Tomiyama, K. Akutsu, and T. Hibi. 1998. A novel ATP-binding cassette transporter involved in multidrug resistance in the phytopathogenic fungus Penicillium digitatum. Appl. Environ. Microbiol. 64:3983-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perea, S., J. L. Lopez-Ribot, W. R. Kirkpatrick, R. K. McAtee, R. A. Santillan, M. Martinez, D. Calabrese, D. Sanglard, and T. F. Patterson. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raeder, U., and P. Broda. 1985. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1:17-20. [Google Scholar]
- 30.Rea, P. A. 1999. MRP subfamily ABC transporters from plants and yeast. J. Exp. Bot. 50:895-913. [Google Scholar]
- 31.Sanglard, D., F. Ischer, and J. Bille. 2001. Role of ATP-binding-cassette transporter genes in high-frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob. Agents Chemother. 45:1174-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73-85. [DOI] [PubMed] [Google Scholar]
- 35.Stehmann, C., and M. A. De Waard. 1995. Accumulation of tebuconazole by isolates of Botrytis cinerea differing in sensitivity to sterol demethylation inhibiting fungicides. Pestic. Sci. 45:311-318. [Google Scholar]
- 36.Stergiopoulos, I., M. M. C. Gielkens, S. D. Goodall, K. Venema, and M. A. De Waard. 2002. Molecular cloning and characterisation of three new ABC transporter encoding genes from the wheat pathogen Mycosphaerella graminicola. Gene 289:141-149. [DOI] [PubMed] [Google Scholar]
- 37.Van den Brink, H. J., H. J. Van Nistelrooy, M. A. De Waard, C. A. Van den Hondel, and R. F. Van Gorcom.1996. Increased resistance to 14 alpha-demethylase inhibitors (DMIs) in Aspergillus niger by coexpression of the Penicillium italicum eburicol 14 alpha-demethylase (cyp51) and the A. niger cytochrome P450 reductase (cprA) genes. J. Biotechnol. 49:13-18. [DOI] [PubMed] [Google Scholar]
- 38.van Tuyl, J. M. 1977. Genetics of fungal resistance to systemic fungicides. Meded Landbouwhogesch Wageningen 77:1-137. [Google Scholar]
- 39.Vermeulen, T., H. Schoonbeek, and M. A. De Waard. 2001. The ABC transporter BcatrB from Botrytis cinerea is a determinant of the phenylpyrrole fungicide fludioxonil. Pestic. Manag. Sci. 57:393-402. [DOI] [PubMed] [Google Scholar]
- 40.Wellmann, H., and K. Schauz. 1993. DMI-resistance in Ustilago maydis. II. Effect of triadimefon on regenerating protoplasts and analysis of fungicide uptake. Pestic. Biochem. Physiol. 46:55-64. [Google Scholar]
- 41.Zwiers, L.-H., and M. A. De Waard. 2000. Characterization of the ABC transporter genes MgAtr1 and MgAtr2 from the wheat pathogen Mycosphaerella graminicola. Fungal Genet. Biol. 30:115-125. [DOI] [PubMed] [Google Scholar]
- 42.Zwiers, L.-H., and M. A. De Waard. 2001. Efficient Agrobacterium tumefaciens-mediated gene disruption in the phytopathogen Mycosphaerella graminicola. Curr. Genet. 39:388-393. [DOI] [PubMed] [Google Scholar]