Abstract
Epidemiological and medical anthropological investigations suggest that flavanol-rich foods exert cardiovascular health benefits. Endothelial dysfunction, a prognostically relevant key event in atherosclerosis, is characterized by a decreased bioactivity of nitric oxide (NO) and impaired flow-mediated vasodilation (FMD). We show in healthy male adults that the ingestion of flavanol-rich cocoa was associated with acute elevations in levels of circulating NO species, an enhanced FMD response of conduit arteries, and an augmented microcirculation. In addition, the concentrations and the chemical profiles of circulating flavanol metabolites were determined, and multivariate regression analyses identified (–)-epicatechin and its metabolite, epicatechin-7-O-glucuronide, as independent predictors of the vascular effects after flavanol-rich cocoa ingestion. A mixture of flavanols/metabolites, resembling the profile and concentration of circulating flavanol compounds in plasma after cocoa ingestion, induced a relaxation in preconstricted rabbit aortic rings ex vivo, thus mimicking acetylcholine-induced relaxations. Ex vivo flavanol-induced relaxation, as well as the in vivo increases in FMD, were abolished by inhibition of NO synthase. Oral administration of chemically pure (–)-epicatechin to humans closely emulated acute vascular effects of flavanol-rich cocoa. Finally, the concept that a chronic intake of high-flavanol diets is associated with prolonged, augmented NO synthesis is supported by data that indicate a correlation between the chronic consumption of a cocoa flavanol-rich diet and the augmented urinary excretion of NO metabolites. Collectively, our data demonstrate that the human ingestion of the flavanol (–)-epicatechin is, at least in part, causally linked to the reported vascular effects observed after the consumption of flavanol-rich cocoa.
Keywords: cardiovascular disease, endothelial function, nitric oxide synthase
Representing one of the most important lifestyle factors, diet can strongly influence the incidence of cardiovascular diseases (CVDs) (1–3). Various phytochemical constituents of certain foods and beverages, in particular a class of compounds called flavanols, have been avidly investigated in recent years. Concurrently, accumulating data from epidemiological investigations indicate an association of an improved cardiovascular prognosis with flavanol-rich diets. Flavanols, such as epicatechin and catechin, and their oligomers, the procyanidins, represent a major class of secondary, polyphenolic plant metabolites. Flavanols are commonly present in most higher plants, and their high content in certain food plants, such as Vitis vinifera (grape wine), Camellia sinensis (tea), and Theobroma cacao (cocoa) are especially noteworthy in the context of human nutrition.
Recent dietary interventions in humans using flavanol-containing foods have substantiated epidemiological data on an inverse relationship between flavanol intake and the risk of CVD, indicating various potential flavanol-mediated bioactivities, including the improvement of vasodilation (4–8), blood pressure (9), insulin resistance, and glucose tolerance (10), the attenuation of platelet reactivity (11), and the improvement of immune responses and antioxidant defense systems (12, 13). Subsequent investigations in vitro were aimed at elucidating the molecular mechanisms that represent the causal mechanistic principles for flavanol-mediated bioactivities in vivo. These results imply that flavanols can affect cellular signaling pathways, modulate cell membrane characteristics and receptor functions, alter the cellular redox environment, and influence gene expression, protein activity, and the metabolic competence of various cell types in culture (14, 15). However, a validation, and subsequent application of the aforementioned knowledge on the bioactivity of flavanols, has not as yet been fully accomplished in the context of human health. The reasons for these shortcomings are, at least in part, based on the fact that food matrices contain a multitude of phytochemical constituents, of which an unknown number may exert physiological effects. Furthermore, despite the fact that previous work structurally characterized major flavanol metabolites in human plasma (16–19), the direct proof that, and which of, the absorbed flavanols and their metabolites are causally linked to the observed cardiovascular effects in humans has yet to be established. Importantly, most data available from in vitro studies are based on the use of flavanol forms as present in foods and not as they exist in circulation, potentially limiting the validity of a subsequent data analysis in the context of human physiology. The extent of this potential drawback becomes apparent when considering that the human metabolism of flavanols gives rise to various O-methylated, O-glucuronidated, and O-sulfated flavanol derivatives that are products of phase I/II biotransformation in intestine and liver (16–19). Flavanols also undergo biotransformation by the gut micro flora resulting in a large variety of phenolic acid derivatives and γ-valerolactone-like compounds (20). However, data on the bioactivity of specific flavanol metabolites are limited.
Several causality criteria for the assessment of any compound as a potential mediator of vascular function have been established previously. Based on the classical criteria of Koch and Dale as applied in the context of cardiovascular research (21, 22), and Hill's causal criteria (23, 24), the following objective mediator criteria have to be met: (i) the test compound should be absorbed by humans, and it should be transported to the appropriate site or tissue, as indicated by its hypothesized effects; (ii) quantitative assessments should indicate that the circulating amounts, and the pharmacokinetics of the test compound absorbed under physiological conditions, are sufficient to mediate the hypothesized effects; (iii) if the test compound is a food constituent, the effect mediated by the pure and chemically identified food constituent should closely mimic physiological effects; (iv) the inhibition of endogenous mediator pathways, hypothesized to be causally linked to the effect exerted by the nutritional test compound, should result in an attenuation of such effects; and (v) the withholding (or loss) of foods containing the test compound, or the withholding of the test compound itself, should be consistent with a reversal or an attenuation of the effect.
To ascertain whether dietary flavanols and their metabolites are vasoactive mediators, we have conducted an investigation using an experimental paradigm that includes the assessment of established surrogate markers of cardiovascular function, and a comprehensive analysis of the circulating nitric oxide (NO) pool and plasma flavanols. The results provide compelling evidence that the flavanol compound (–)-epicatechin mediates, at least in part, the beneficial vascular effects associated with the consumption of flavanol-rich cocoa in humans. Potentially of more significance, the results reported in this paper provide a mechanistic underpinning for the epidemiological observation of an inverse association between the consumption of diets rich in plant foods and the risk for CVD.
Results
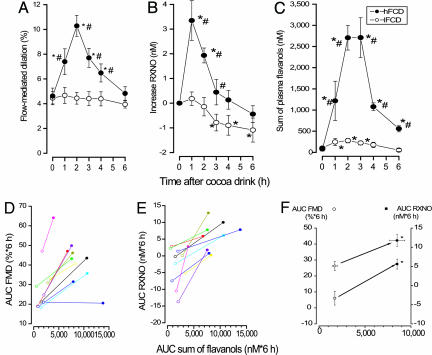
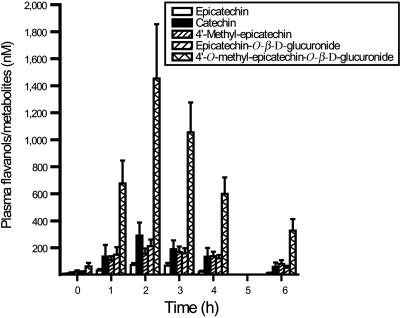
Time Course of Acute Vascular Effects and Flavanol Metabolites. To characterize the acute cardiovascular effects that result from the consumption of flavanol-rich cocoa, we conducted a dietary intervention study using cocoa drinks with a high or low flavanol content. The oral ingestion of the high-flavanol cocoa drink (hFCD), but not that of the low-flavanol cocoa drink (lFCD), resulted in a significant transient increase of the FMD response at 1–4 h postconsumption compared with baseline of the same day and the respective time points of lFCD treatments (Fig. 1A). The hFCD-mediated increase in FMD was paralleled by an augmentation of plasma nitroso species (RXNO) concentrations at 1–3 h (Fig. 1B). Measurements of the peripheral arterial tonometry (PAT) index also demonstrated significantly increased responses after the ingestion of a hFCD as compared with a lFCD. Based on the maximal PAT responses, the ingestion of a hFCD resulted in a 68.9 ± 10.3% augmentation of the PAT index as compared with an increase of 18.1 ± 3.3% after a lFCD (n = 6; P = 0.004). The mean PAT index at baseline, as calculated by using all baseline measurements, was 1.9 ± 0.1 (n = 12). Moreover, consumption of the hFCD significantly increased the sum of determined circulating flavanols/metabolites as compared with the lFCD (Fig. 1C). Fig. 2 depicts the pharmacokinetics for major compounds after the ingestion of the hFCD. The temporal kinetics of the increase in plasma flavanols/metabolites was similar to those of FMD, PAT, and RXNO (Fig. 1 A–C).
Fig. 1.
Time courses of FMD, RXNO, and total circulating flavanols. (A–C) Ingestion of a hFCD (917 mg of total flavanols; filled circles) exerted significant increases in FMD (A), RXNO (B), and circulating flavanols (C) compared with a low-flavanol control drink (lFCD = 37 mg; open circles; n = 10). lFCD ingestion slightly increased the sum of circulating flavanols (C) but had no effect on FMD and RXNO (mean values ± SEM). (D–F) AUCs of FMD, RXNO, and sum of circulating flavanols after ingestion of a lFCD (open symbols) or a hFCD (filled symbols). AUC of flavanol plasma concentration increased in all volunteers; in 9 of 10 individuals this was paralleled by an increase in AUC of FMD. (F) Mean values for AUCs of FMD (circles), RXNO (squares), and sum of circulating flavanols are significantly higher after hFCD (calculated from individual values presented in D and E). *, P < 0.05 vs. baseline at 0 h of respective day; #, P < 0.05 vs. respective time point on control day.
Fig. 2.
Plasma profile of flavanols/metabolites. Time course of epicatechin, catechin, and selected postabsorption metabolites after ingestion of a high-flavanol (917 mg) cocoa drink (see squares in Fig. 1C). Values depicted are means ± SEM. *, P < 0.05 vs. baseline at 0 h.
To further evaluate a dose-dependence between changes in plasma flavanols and vascular function, and to control for individual variations in absorption, we analyzed the individual areas under the curve (AUCs) of FMD and RXNO plasma levels against the AUCs of the individual sum of plasma flavanols/metabolites, respectively (Fig. 1 D–F). In all volunteers, consumption of the hFCD resulted in increases of plasma flavanols/metabolites that were paralleled by an augmentation of RXNO levels. In 9 of 10 volunteers, individual increases in plasma flavanol/metabolite and RXNO levels coincided with an amplified FMD response. PAT measurements demonstrated that the AUC of the PAT index was increased by 25.1 ± 3.7% (n = 6, P < 0.001) from a baseline of 1.9 ± 0.1 (n = 12) after the ingestion of a hFCD as compared with a lFCD.
Circulating Flavanols/Metabolites Predict FMD. We observed significant univariate correlations between flavanols/metabolites and FMD (4′-O-methyl-epicatechin-O-β-d-glucuronide, r = 0.402; 4′-O-methyl-epicatechin, r = 0.421; epicatechin-7-O-β-d-glucuronide, r = 0.450; epicatechin, r = 0.486; sum of flavanols, r = 0.432; P < 0.01 each). Significance was not observed for catechin. In a multivariate regression analysis including all determined circulating flavanols/metabolites, only epicatechin and its metabolite epicatechin-7-O-glucuronide predicted the magnitude of FMD (P < 0.01 and P < 0.05, respectively; R2 = 0.31 for the model), suggesting that epicatechin is, at least in part, directly, and causally, involved in the observed improvement of vascular function.
Attenuation of Effects by NO Synthase (NOS) Inhibition. The potential dependence on NO of the hFCD-mediated increase in FMD was investigated in a subgroup of volunteers (n = 3) using an i.v. infusion of the NOS inhibitor l-NG-mono-methyl-arginine (L-NMMA). Our data show that the FMD response at 2 h after a hFCD ingestion equaled 9.2 ± 0.4% in the absence of L-NMMA. In comparison, subsequent assessments using continued L-NMMA infusions in the selfsame subgroup of volunteers showed a greatly reduced FMD response of 0.6 ± 0.4% (P < 0.01) at 2 h after a hFCD ingestion.
Ex Vivo Vasodilation. A causal relationship between circulating flavanols/metabolites and NO-dependent vasodilation was further substantiated by using isolated rabbit aortic rings ex vivo. The incubation of preconstricted aortic rings with a mixture of flavanols/metabolites that mimicked the chemical composition of flavanols/metabolites, as well as the mean plasma concentration, as determined 2 h after ingestion of a hFCD (Fig. 2), resulted in a relaxation. The maximal relaxation induced by the flavanol/metabolite mix was 74.2 ± 14.5% (n = 3; 10 mM acetylcholine induced a maximal relaxation of 83.6 ± 3.6%; 100% = conditions before constriction using norepinephrine). Parallel treatments of preconstricted aortic rings with the flavanol/metabolite mix, or acetylcholine, in the presence of L-NMMA did not result in relaxation.
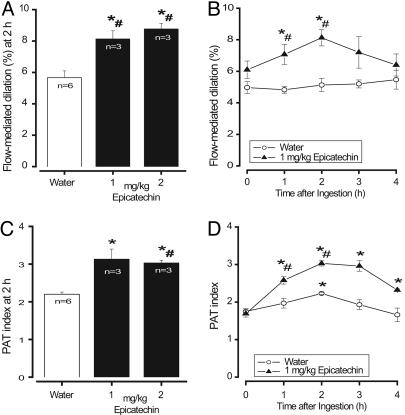
Isolated, Chemically Pure Epicatechin Increases Vascular Function. Given the results of our multivariate regression analysis that identified circulating epicatechin and epicatechin-7-O-glucuronide as predictors for a modulation of vascular function, we conducted a “proof-of-concept” study, during which we orally administered pure (–)-epicatechin to healthy volunteers. The doses administered were based on the epicatechin content of the hFCD. Two hours after the oral ingestion of (–)-epicatechin at doses of 1 or 2 mg/kg of body weight (BW) dissolved in water, all volunteers had significantly increased FMD and PAT responses, whereas the ingestion of water alone had no effects on FMD or PAT (Fig. 3). The responses measured 2 h after ingestion of (–)-epicatechin showed no statistical differences between the two doses tested (Fig. 3 A and B). Fig. 3 B and D illustrates the kinetics of the FMD and PAT responses after the oral ingestion of (–)-epicatechin at 1 mg/kg of BW demonstrating significantly increased values as compared with baseline, as well as compared with water controls. The magnitude and kinetics of changes in vascular function were similar to the effects observed after ingestion of hFCD.
Fig. 3.
Proof-of-concept: Vascular response after oral ingestion of (–)-epicatechin. (A and C) FMD (A) and PAT (C) index significantly increased 2 h after ingestion of 1 or 2 mg/kg epicatechin in water (filled columns) but not water alone (open column; n = 3; cross-over). (B and D) Time course of FMD (B) and PAT (D) index after ingestion of water (open circles) or 1 mg/kg (–)-epicatechin in water (n = 3). Data represent means ± SEM. *, P < 0.05 vs. baseline at 0 h of respective day; #, P < 0.05 vs. respective time point on control day.
Flavanol Consumption in Kuna Indians. As an initial test of whether or not the chronic consumption of a high-flavanol diet is associated with a persistent augmentation of NO-production, we studied two genetically closely related populations of Kuna Indians. Indigenous Kuna Indians, who live in the San Blas islands off the coast of Panama, represent a very propitious study population because they have only minimal increases in blood pressure with age, and hypertension and other CVDs are rare. The factors involved are primarily environmental, rather than genetic, because this protection is lost upon migration to mainland Panama City (25). One contributing factor seems to be dietary because the Kuna living on San Blas customarily consume large amounts of flavanol-rich cocoa (26). In the current study, urine samples collected from a subset of individuals, who were partaking in a larger study aimed at investigating diet-blood pressure correlations in the Kuna (27), were analyzed for flavanol metabolites and nitrite+nitrate concentrations. All volunteers partaking in this study where matched for age, gender, and weight. Island-dwelling Kuna consumed an average of 3–4 cups of cocoa per day. Those living on the mainland consumed on average <4 cups per week. The cocoa powder used on the island contained 0.196 g of total procyanidins per g (33). Reasonably assuming that the cocoa beverage contained an average of 10 g of cocoa powder per 100 ml of fluid, and defining the average volume of a cup to be 100 ml, the typical daily procyanidin intake on the island was in the range of 600–900 mg. Consistent with the above estimates, urinary levels of flavanol metabolites, expressed as epicatechin equivalents, are more than six times higher in island dwellers than in mainland inhabitants (Fig. 4, which is published as supporting information on the PNAS web site). Although the dietary records predicted a higher urinary nitrate+nitrite level in mainland inhabitants as compared with island dwellers, the sum of urinary nitrite+nitrate levels in island dwellers (n = 16) is more than twice that of Kuna living on the mainland (n = 18) (Fig. 4).
Discussion
Based on the above causality criteria (28), the data sets that emanated from this investigation establish the following. (i) Flavanols are absorbed from a food matrix, they are quantifiable in circulation, and the kinetics of their appearance in plasma temporally parallels the vascular effects observed. (ii) The circulating concentrations of flavanols in plasma are sufficient to mediate ex vivo vasodilation in isolated aortic rings, mimicking a physiological function of the endogenous mediator acetylcholine. (iii) Pure epicatechin ingested by humans closely and quantitatively mimics the vascular effects of flavanol-rich cocoa. (iv) The direct inhibition of NOS in humans, and in isolated aortic rings, abolishes vascular effects. (v) Chronic consumption of a high-flavanol diet is associated with a high urinary excretion of NO metabolites and consistent with an augmented NO production.
Analysis of Circulating Flavonoids. A main characteristic of this investigation is the simultaneous assessment of surrogate markers of vascular function and circulating flavanols/metabolites. The synthesis of authentic flavanol metabolites, previously identified in humans as major phase I/II biotransformation products of flavanols (16–18), has made it possible to optimize and validate plasma extraction and chromatography procedures with regard to the individual compounds analyzed here. Nonetheless, the following limitations should be taken into consideration when interpreting the data presented. (i) Although the compounds selected for analysis represent most of the abundant metabolites, the complete flavanol metabolite profile of any individual contains additional metabolites that were not assessed here. (ii) The presence or absence and the time-dependent appearance and disappearance of a specific flavanol metabolite in plasma can vary significantly among individuals, especially with regard to some of the less abundant forms. (iii) Initial investigations into the binding/association of flavanols/metabolites to human erythrocytes in vivo indicated that the overall concentration of circulating flavanol forms is likely to be significantly higher as compared with the levels in plasma alone. It has to be also noted that we have omitted sulfated flavanol derivatives from our investigations. This omission was based on previous work and preliminary data demonstrating their structural heterogeneity (i.e., various O-sulfation products of epicatechin, methylated epicatechin, glucuronidated epicatechin, and methylated+glucuronidated epicatechin), considerable variations among individuals, and their (as based on structurally distinct sulfates) relatively low abundance. In addition, as depicted in Fig. 2, the most abundant metabolites determined after the ingestion of f lavanol-rich cocoa were 4′-O-methyl-epicatechin-O-β-d-glucuronides. In agreement with previously published data (16), and based on authentic standards as well as verifying analytical procedures (please refer to Supporting Materials and Methods, which is published as supporting information on the PNAS web site), we have identified various isomers of 4′-O-methyl-epicatechin-O-β-d-glucuronide with glucuronidation in positions 7, 5, and 3′ of the flavanol structure, of which derivatizations in positions 7 and 5 were more common. For the purpose of this investigation, we have not distinguished between structurally distinct isomers of 4′-O-methyl-epicatechin-O-β-d-glucuronide. In summary, our analyses comprised most of the high-abundance and commonly found flavanol metabolites after cocoa consumption. In contrast to some of the less abundant metabolites, all of the evaluated compounds were present in circulation of every volunteer participating. Based on these observations, and the ex vivo data on the metabolite mix-induced relaxation of preconstricted aortic rings, it can be argued that the group of evaluated flavanol derivatives represents a bioactive circulating flavanol pool, although other flavanol or procyanidin constituents of foods/beverages may exert additional bioactivities.
Effects of Flavanol-Rich Foods on Vascular Function and NO Synthesis. Traditional cardiovascular risk factors are diverse and include smoking, aging (29), hypercholesterolemia, hypertension, hyperglycemia (30), and a family history of premature atherosclerotic disease. All of those risk factors are associated with attenuation/loss of endothelium-dependent vasodilation. Many published studies involving 2,000 or more patients with atherosclerosis have proven the prognostic value of endothelial vasomotor dysfunction (31). In this context, FMD represents the current gold standard for the noninvasive measurement of endothelial function in humans. Regarding dietary interventions, various studies previously reported acute and chronic increases in endothelium-dependent vasodilation after the ingestion of flavanol-rich foods/beverages such as red wine (32), purple grape juice (7), tea (33), cocoa (4, 8), and chocolate (34). In most but not in all studies (4), endothelium-dependent vasodilation was measured as FMD by using methodologies comparable to those applied here. Results that emanated from these studies corroborate the hypothesis that an increase in NO bioactivity represents the common underlying mechanistic principle for augmented vasodilation. Because of the fact that FMD is almost exclusively mediated by NO (35), increases in NOS activity are principally paralleled by increases in plasma nitroso species (36), and increased vasodilation can be reversed by using NOS inhibition (4, 37). Thus, parallel measurements of FMD and circulating NO species, in conjunction with NOS inhibition studies, represent a mechanistically strong experimental framework. In our current study, we measured parallel time courses of individual flavanol metabolites, FMD, and NO metabolites after the oral ingestion of flavanol-rich cocoa drinks, and thus established a causal link between the flavanol compounds present in a dietary matrix, their appearance in circulation, and an increased FMD. To prove that it is indeed a flavanol that is the causative principle responsible for the observed increase in vascular function, we demonstrated that the ingestion of pure epicatechin mimicked the vascular effects observed after flavanol-rich cocoa consumption, and that the flavanols present in plasma at 2 h after ingestion of the flavanol-rich cocoa drink caused a relaxation of preconstricted aortic rings. These effects were abolished in vivo and ex vivo by inhibiting NOS. To underscore the concept of a flavanol-mediated increase in NO production, we showed that the loss of flavanol-rich foods, i.e., the exchange of a flavanol-rich, cocoa-based diet for a Western diet, is associated with a reduction in urinary nitrate+nitrite excretion in urbanized Kuna Indians. Consistent with the current understanding of the role of NO in CVD, Kuna Indians who moved from the island to the mainland are characterized by a higher incidence in CVDs, especially hypertension.
Future Directions. To establish the relevance of these findings for a greater and more general population, it is necessary to apply similar study paradigms to populations with greater diversity in terms of genetics, age, and medical history. Furthermore, the scope of this investigation was the characterization of acute cardiovascular effects and the identification of causative, active compounds. Thus, a direct line of evidence for the efficacy of chronic flavanol consumption remains to be established.
In this context, it should be noted that most other flavanol-rich foods also contain relatively high amounts of epicatechin, despite the fact that their overall flavanol profile can vary greatly. Our findings, in conjunction with the reported outcomes of dietary interventions using different flavanol-rich foods (5–7), support the notion that epicatechin is, indeed, an important mediator of the cardiovascular effects of diets rich in flavanols. Given the above, it is reasonable to speculate that the reported positive cardiovascular effects of other flavanol-rich foods/beverages, including purple grape juice (7), tea (5), and red wine (6), are mediated in part through similar mechanisms. Thus, the findings in the current paper may provide new avenues to dietary or therapeutic interventions aimed at improving, and maintaining, cardiovascular health. From a broader perspective, the data presented provide a mechanistic underpinning to the hypothesis that diets rich in plant foods are associated with a reduced risk for vascular disease (38). Although it has been argued that the inverse relationship between plant-based diets and CVD may be the result of the absence of dietary components associated with vascular risk (e.g., high fat and high cholesterol) in such diets, our data support the notion that it is, at least in part, the presence of beneficial components, such as flavanols, that drive these positive inverse correlations. A determination of the epicatechin equivalents, perhaps defined as the ability of a food or beverage, on a caloric or per serving basis, to improve surrogate markers for CVD, could provide a novel means for evaluating the potential cardiovascular health benefit of plant-based foods.
Materials and Methods
To apply the five causality criteria detailed above, and thus to characterize a food constituent as a bioactive mediator of beneficial vascular effects, the following was undertaken. (i) We measured levels of plasma flavanols/metabolites, endothelial function by FMD, and plasma NO metabolites after ingestion of cocoa drinks with high or low flavanol content in healthy volunteers. FMD of the brachial artery is a noninvasive measurement of endothelial function, it represents a functional NO readout, and it is an established surrogate marker for cardiovascular events. Reactive hyperemia-induced PAT measurements were used to assess microvascular function. RXNO were used as a biochemical marker for NO. To correlate measured effects with specific compounds present in circulation, we measured plasma levels of structurally related phase I/II metabolites of flavanols. Although we identified various circulating O-glucuronidated, O-methylated, and O-sulfated flavanol derivatives in the course of this investigation (Fig. 5, which is published as supporting information on the PNAS web site), we focused our quantitative assessments on 4′-O-methyl-epicatechin-O-β-d-glucuronide (1) (we did not differentiate between a glucuronidation at positions 7, 5, or 3′ on the epicatechin ring structure), 4′-O-methyl-epicatechin (2), epicatechin-7-O-β-d-glucuronide (3), catechin (4), and epicatechin (5). (ii) We investigated ex vivo vasodilation of isolated rabbit aortic rings using a flavanol metabolite mix that resembled the flavanol profile/concentration observed in human plasma after the consumption of flavanol-rich cocoa. (iii) We administered pure epicatechin or water and measured FMD in healthy volunteers. (iv) A cocoa drink with high flavanol content was administered during systemic NOS inhibition. (v) Urinary NO and flavanol metabolites were measured in indigenous Kuna Indians with habitually high cocoa flavanol intakes, and in tribesmen from the Kuna islands who had moved to the suburbs of Panama City and who were characterized by low cocoa intakes.
Human Studies. We administered cocoa and pure flavanols to healthy male subjects without arterial hypertension, hypercholesterolemia, diabetes mellitus, acute inflammation, or dietary supplement use [males aged 25–32 years with a body mass index of 19–23 (calculated as weight in kilograms divided by the square of height in meters); n = 16]. Volunteers fasted overnight for at least 12 h before beginning and during the entire assessment period. No further dietary restrictions or recommendations were imposed. All protocols were approved by the ethics board of the Heinrich-Heine University.
Flavanol Cocoa Drinks. In a randomized, double-blind, cross-over design, we administered two powdered cocoa drinks mixed in 300 ml of water with a high or low flavanol content (hFCD = 917 mg and lFCD = 37 mg of total flavanols, respectively; CocoaPro cocoa powder, Mars Incorporated) on two separate days with wash-out periods of >2 days in between (n = 10). FMD, RXNO, and flavanols/metabolites were measured before as well as 1, 2, 3, 4, and 6 h after ingestion of the drinks.
Pure Flavanol. In a randomized cross-over study, healthy individuals received water or (–)-epicatechin dissolved in water (3 ml/kg of BW; n = 6; water at room temperature). The (–)-epicatechin doses were 1 or 2 mg/kg of BW (each n = 3). FMD was measured before and 1, 2, 3, and 4 h (1 mg/kg) or 2 h (2 mg/kg) after cross-over application of epicatechin in water or water only, respectively. Investigators undertaking oral supplementations, FMD/PAT measurements, and sample analysis (RXNO, flavanols/metabolites) were blinded with regard to treatment and sample information.
Flavanol-Rich Cocoa Drinks Under NOS Inhibition. We assessed NOS dependence in a subgroup of three volunteers. An hFCD was administered with or without systemic NOS-inhibition. NOS-inhibition was achieved by i.v. infusion of L-NMMA (loading dose of 1 mg·kg–1·min–1 over 3 min, maintenance dose of 0.2 mg·kg–1·min–1) 30 min before, and until 2 h after, ingestion of a hFCD.
Kuna Indians. Urine was collected from both island-dwelling (n = 16) and mainland (n = 18) Kuna Indians. The volunteers represent a subset of a larger study population that was recruited to further investigate the relationship between diet and blood pressure in the Kuna Indians (27). Dietary assessments and urine collections were approved by the ethics committee of the Brigham and Women's Hospital, the Panamanian Ministry of Health, and the Council of the Kuna Elders.
Ex Vivo Aortic Ring Experiments. Experiments were carried out by using isolated rabbit (New Zealand Whites) aortic rings as detailed in ref. 39. We controlled for artifacts that might be caused by hydrogen peroxide, a product of flavanol decomposition in buffers and media (40), by adding catalase (400 units/ml) into the tissue bath.
Test Drinks. For kinetic studies, we used a cocoa beverage powder (CocoaPro, Mars Incorporated) with a high or a low flavanol content (hFCD = 917 mg and lFCD = 37 mg of total flavanols, respectively; 19% of total flavanols = epicatechin) mixed in 300 ml of water. Both drinks were similar in taste, indistinguishable by color or packaging, matched for macronutrients and calories, and adjusted for equal contents of theobromine and caffeine. Pure (–)-epicatechin was provided by Mars Incorporated. All isolation procedures were carried out under food-grade conditions according to US 21 CFR Part 110 using food-grade materials. The compound was tested for its chemical purity by using HPLC. The chemical purity of the (–)-epicatechin was >99%. Epicatechin was dissolved in 300 ml of water before administration in drinks.
Assessment of Vascular Function. FMD was measured as described in refs. 28 and 41. Reactive hyperemia was induced by 5 min of distal lower arm occlusion. Sixty seconds after release of occlusion, the arterial diameter was assessed and FMD was calculated as relative diameter gain compared with baseline. In parallel experiments (the same arm, at identical time points), we determined microvascular function by PAT index as described previously, using the contralateral arm to control for potentially confounding sympathetic effects (4, 42). PAT measurements were obtained by using an Endo-PAT2000 (Itamar Medical).
Analysis of Plasma Nitroso Species. Plasma levels of the circulating NO pool (RXNO: sum of S-, N-nitrosothiols and iron-nitrosyl complexes) were determined by using a gas-phase chemiluminescence assay as described in ref. 36. RXNO analyses were carried out immediately after blood draws.
Analysis of Urinary Nitrite/Nitrate. Twenty-four-hour urine samples from the two Kuna populations were collected in vessels and kept on dry ice throughout the collection period. All samples were stored below –40°C and analyzed as detailed in ref. 43.
Quantification of Circulating Flavanols/Metabolites. Authentic standards for flavanols/metabolites [4′-O-methyl-epicatechin-7-O-β-d-glucuronide (1), 4′-O-methyl-epicatechin (2), 3′-O-methyl-epicatechin-5/7-O-β-d-glucuronide (3), 3′-O-methyl-epicatechin (4), epicatechin-7-O-β-d-glucuronide (5), epicatechin (6), catechin (7), 4′-O-methyl-catechin (8), and 3′-O-methyl-catechin (9)] were provided by Mars Incorporated. 3′-O-ethyl-epicatechin (3′Eth-ylEC), a not naturally occurring epicatechin derivative (provided by Mars Incorporated), was used as the internal standard. Structural identity of the standards was verified by mass spectroscopy (Figs. 6 and 7, which are published as supporting information on the PNAS web site) and NMR spectroscopy (for additional information, see Tables 1 and 2 and Supporting Materials and Methods, which are published as supporting information on the PNAS web site). Throughout these investigations, the analytical chemists were blinded with regard to sample information. For a detailed description of sample preparations, HPLC analyses (Fig. 5), and method validation, please refer to Supporting Materials and Methods.
Statistical Analyses. Data are presented as mean ± SEM. Statistical analyses of time courses were performed by using repeated-measurements ANOVA with two within-subject factors (high/low-flavanol drink and time after ingestion). Pairwise comparisons were made for the simple effects of session using Bonferroni correction for multiple comparisons. The means for the interaction are shown in Fig. 1 A–C. Univariate correlations were calculated by using Pearson's r. A multivariate regression analysis was performed to identify individual plasma flavanols/metabolites as independent predictors for FMD. Statistical significance was assumed if a null hypothesis could be rejected at P = 0.05. The AUC, as presented in Fig. 1 D–F, were calculated by using the trapezoidal model. All analyses were performed with spss 11.0.1 (SPSS, Chicago) and origin 7 (OriginLab).
Supplementary Material
Acknowledgments
We thank D. Finis, P. Brouzos, and S. Matern for technical assistance and quality controls (all of the University of Duesseldorf); R. R. Holt for aortic ring testing; Dr. H. B. Gross, T. J. Orozco, and J. L. Ensunsa for sample preparation and HPLC analysis (all of the University of California, Davis); and Dr. Mark Kelm (Mars Incorporated) for work related to the structural identification/verification of authentic flavanol metabolite standards. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 575 and 612), Biomedizinisches Forschungszentrum of the University of Duesseldorf, and Mars Incorporated. H. Sies is a Fellow of the National Foundation for Cancer Research.
Conflict of interest statement: H.H.S. and C.K.-U. are employed by Mars Incorporated, and Mars Incorporated has, in part, financially supported this work.
Abbreviations: AUC, area under the curve; CVD, cardiovascular disease; FMD, flow-mediated dilation; hFCD, high-flavanol cocoa drink; lFCD, low-flavanol cocoa drink; L-NMMA, l-NG-mono-methyl-arginine; NOS, NO synthase; PAT, peripheral arterial tonometry; RXNO, plasma nitroso species (sum of nitrosated and nitrosylated).
References
- 1.Parikh, P., McDaniel, M. C., Ashen, M. D., Miller, J. I., Sorrentino, M., Chan, V., Blumenthal, R. S. & Sperling, L. S. (2005) J. Am. Coll. Cardiol. 45, 1379–1387. [DOI] [PubMed] [Google Scholar]
- 2.Graf, B. A., Milbury, P. E. & Blumberg, J. B. (2005) J. Med. Food 8, 281–290. [DOI] [PubMed] [Google Scholar]
- 3.Arts, I. C. & Hollman, P. C. (2005) Am. J. Clin. Nutr. 81, 317S–325S. [DOI] [PubMed] [Google Scholar]
- 4.Fisher, N. D., Hughes, M., Gerhard-Herman, M. & Hollenberg, N. K. (2003) J. Hypertens. 21, 2281–2286. [DOI] [PubMed] [Google Scholar]
- 5.Hirata, K., Shimada, K., Watanabe, H., Otsuka, R., Tokai, K., Yoshiyama, M., Homma, S. & Yoshikawa, J. (2004) Am. J. Cardiol. 93, 1384–1388. [DOI] [PubMed] [Google Scholar]
- 6.Janszky, I., Ericson, M., Blom, M., Georgiades, A., Magnusson, J. O., Alinagizadeh, H. & Ahnve, S. (2005) Heart 91, 314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein, J. H., Keevil, J. G., Wiebe, D. A., Aeschlimann, S. & Folts, J. D. (1999) Circulation 100, 1050–1055. [DOI] [PubMed] [Google Scholar]
- 8.Heiss, C., Dejam, A., Kleinbongard, P., Schewe, T., Sies, H. & Kelm, M. (2003) J. Am. Med. Assoc. 290, 1030–1031. [DOI] [PubMed] [Google Scholar]
- 9.Taubert, D., Berkels, R., Roesen, R. & Klaus, W. (2003) J. Am. Med. Assoc. 290, 1029–1030. [DOI] [PubMed] [Google Scholar]
- 10.Grassi, D., Lippi, C., Necozione, S., Desideri, G. & Ferri, C. (2005) Am. J. Clin. Nutr. 81, 611–614. [DOI] [PubMed] [Google Scholar]
- 11.Holt, R. R., Schramm, D. D., Keen, C. L., Lazarus, S. A. & Schmitz, H. H. (2002) J. Am. Med. Assoc. 287, 2212–2213. [DOI] [PubMed] [Google Scholar]
- 12.Sies, H., Schewe, T., Heiss, C. & Kelm, M. (2005) Am. J. Clin. Nutr. 81, 304S–312S. [DOI] [PubMed] [Google Scholar]
- 13.Keen, C. L., Holt, R. R., Oteiza, P. I., Fraga, C. G. & Schmitz, H. H. (2005) Am. J. Clin. Nutr. 81, 298S–303S. [DOI] [PubMed] [Google Scholar]
- 14.Scalbert, A., Manach, C., Morand, C., Remesy, C. & Jimenez, L. (2005) Crit. Rev. Food Sci. Nutr. 45, 287–306. [DOI] [PubMed] [Google Scholar]
- 15.Middleton, E., Jr., Kandaswami, C. & Theoharides, T. C. (2000) Pharmacol. Rev. 52, 673–751. [PubMed] [Google Scholar]
- 16.Natsume, M., Osakabe, N., Oyama, M., Sasaki, M., Baba, S., Nakamura, Y., Osawa, T. & Terao, J. (2003) Free Radical Biol. Med. 34, 840–849. [DOI] [PubMed] [Google Scholar]
- 17.Hackett, A. M., Griffiths, L. A., Broillet, A. & Wermeille, M. (1983) Xenobiotica (London) 13, 279–286. [DOI] [PubMed] [Google Scholar]
- 18.Kuhnle, G., Spencer, J. P., Schroeter, H., Shenoy, B., Debnam, E. S., Srai, S. K., Rice-Evans, C. & Hahn, U. (2000) Biochem. Biophys. Res. Commun. 277, 507–512. [DOI] [PubMed] [Google Scholar]
- 19.Schroeter, H., Holt, R. R., Orozco, T. J., Schmitz, H. H. & Keen, C. L. (2003) Nature 426, 787–788. [DOI] [PubMed] [Google Scholar]
- 20.Rios, L. Y., Gonthier, M. P., Remesy, C., Mila, I., Lapierre, C., Lazarus, S. A., Williamson, G. & Scalbert, A. (2003) Am. J. Clin. Nutr. 77, 912–918. [DOI] [PubMed] [Google Scholar]
- 21.Kelm, M. & Schrader, J. (1990) Circ. Res. 66, 1561–1575. [DOI] [PubMed] [Google Scholar]
- 22.Feigl, E. O. (1983) Physiol. Rev. 63, 1–205. [DOI] [PubMed] [Google Scholar]
- 23.Hill, A. B. (1965) Proc. R. Soc. Med. 58, 295–300. [PMC free article] [PubMed] [Google Scholar]
- 24.Thygesen, L. C., Andersen, G. S. & Andersen, H. (2005) J. Epidemiol. Community Health 59, 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollenberg, N. K., Martinez, G., McCullough, M., Meinking, T., Passan, D., Preston, M., Rivera, A., Taplin, D. & Vicaria-Clement, M. (1997) Hypertension 29, 171–176. [DOI] [PubMed] [Google Scholar]
- 26.Chevaux, K. A., Jackson, L., Villar, M. E., Mundi, J. A., Commisso, J. F. & Adamson, G. E. (2001) J. Food Compos. Anal. 14, 553–563. [Google Scholar]
- 27.McCullough, M. L., Chevaux, K. A., Jackson, L., Preston, M., Matinez, G., Schmitz, H., Coletti, C., Campos, H. & Hollenberg, N. K. (2005) J. Vasc. Pharmacol., in press. [DOI] [PubMed]
- 28.Preik, M., Lauer, T., Heiss, C., Tabery, S., Strauer, B. E. & Kelm, M. (2000) Ultraschall Med. 21, 195–198. [DOI] [PubMed] [Google Scholar]
- 29.Heiss, C., Keymel, S., Niesler, U., Ziemann, J., Kelm, M. & Kalka, C. (2005) J. Am. Coll. Cardiol. 45, 1441–1448. [DOI] [PubMed] [Google Scholar]
- 30.Sies, H., Stahl, W. & Sevanian, A. (2005) J. Nutr. 135, 969–972. [DOI] [PubMed] [Google Scholar]
- 31.Widlansky, M. E., Gokce, N., Keaney, J. F., Jr., & Vita, J. A. (2003) J. Am. Coll. Cardiol. 42, 1149–1160. [DOI] [PubMed] [Google Scholar]
- 32.Agewall, S., Wright, S., Doughty, R. N., Whalley, G. A., Duxbury, M. & Sharpe, N. (2000) Eur. Heart J. 21, 74–78. [DOI] [PubMed] [Google Scholar]
- 33.Duffy, S. J., Keaney, J. F., Jr., Holbrook, M., Gokce, N., Swerdloff, P. L., Frei, B. & Vita, J. A. (2001) Circulation 104, 151–156. [DOI] [PubMed] [Google Scholar]
- 34.Engler, M. B., Engler, M. M., Chen, C. Y., Malloy, M. J., Browne, A., Chiu, E. Y., Kwak, H. K., Milbury, P., Paul, S. M., Blumberg, J., et al. (2004) J. Am. Coll. Nutr. 23, 197–204. [DOI] [PubMed] [Google Scholar]
- 35.Joannides, R., Haefeli, W. E., Lindner, L., Richard, V., Bakkali, E. H., Thuillez, C. & Lüscher, T. F. (1995) Circulation 91, 1314–1319. [DOI] [PubMed] [Google Scholar]
- 36.Rassaf, T., Preik, M., Kleinbongard, P., Lauer, T., Heiss, C., Strauer, B. E., Feelisch, M. & Kelm, M. (2002) J. Clin. Invest. 109, 1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heiss, C., Kleinbongard, P., Dejam, A., Perré, S., Schroeter, H., Sies, H. & Kelm, M. (2005) J. Am. Coll. Cardiol. 46, 1276–1283. [DOI] [PubMed] [Google Scholar]
- 38.Hung, H. C., Joshipura, K. J., Jiang, R., Hu, F. B., Hunter, D., Smith-Warner, S. A., Colditz, G. A., Rosner, B., Spiegelman, D. & Willett, W. C. (2004) J. Natl. Cancer Inst. 96, 1577–1584. [DOI] [PubMed] [Google Scholar]
- 39.Karim, M., McCormick, K. & Kappagoda, C. T. (2000) J. Nutr. 130, 2105S–2108S. [DOI] [PubMed] [Google Scholar]
- 40.Long, L. H., Clement, M. V. & Halliwell, B. (2000) Biochem. Biophys. Res. Commun. 273, 50–53. [DOI] [PubMed] [Google Scholar]
- 41.Corretti, M. C., Anderson, T. J., Benjamin, E. J., Celermajer, D., Charbonneau, F., Creager, M. A., Deanfield, J., Drexler, H., Gerhard-Herman, M., Herrington, D., et al. (2002) J. Am. Coll. Cardiol. 39, 257–265. [DOI] [PubMed] [Google Scholar]
- 42.Bonetti, P. O., Pumper, G. M., Higano, S. T., Holmes, D. R., Jr., Kuvin, J. T. & Lerman, A. (2004) J Am. Coll. Cardiol. 44, 2137–2141. [DOI] [PubMed] [Google Scholar]
- 43.Brien, J. F., McLaughlin, B. E., Nakatsu, K. & Marks, G. S. (1996) Methods Enzymol. 268, 83–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.