Abstract
Artemisinin-derivative combination therapies (ACT) are highly efficacious against multidrug-resistant Plasmodium falciparum malaria. Few efficacy data, however, are available for vivax malaria. With high rates of chloroquine (CQ) resistance in both vivax and falciparum malaria in Papua Province, Indonesia, new combination therapies are required for both species. We recently found artesunate plus sulfadoxine-pyrimethamine (ART-SP) to be highly effective (96%) in the treatment of falciparum malaria in Papua Province. Following a preliminary study of CQ plus sulfadoxine-pyrimethamine (CQ-SP) for the treatment of Plasmodium vivax infection, we used modified World Health Organization criteria to evaluate the efficacy of ART-SP for the treatment of vivax malaria in Papua. Nineteen of 22 patients treated with ART-SP could be evaluated on day 28, with no early treatment failures. Adequate clinical and parasitological responses were found by day 14 in all 20 (100%) of the patients able to be evaluated and by day 28 in 17 patients (89.5%). Fever and parasite clearance times were short, with hematological improvement observed in 70.6% of the patients. Double (at positions 58 and 117) and quadruple (at positions 57, 58, 61, and 117) mutations in the P. vivax dihydrofolate reductase (PvDHFR) were common in Papuan P. vivax isolates (46 and 18%, respectively). Treatment failure with SP-containing regimens was significantly higher with isolates with this PvDHFR quadruple mutation, which included a novel T→M mutation at residue 61 linked to an S→T (but not an S→N) mutation at residue 117. ART-SP ACT resulted in a high cure rate for both major Plasmodium species in Papua, though progression of DHFR mutations in both species due to the continued use of SP monotherapy for clinically diagnosed malaria threatens the future utility of this combination.
High rates of chloroquine (CQ) resistance and CQ treatment failure have been found in both vivax and falciparum malaria in Papua Province (formerly Irian Jaya), Indonesia (2, 3, 5, 21, 28, 38), and new combination therapies are required for each species. Artemisinin-derivative combination therapies (ACT) are being used increasingly for the treatment of multidrug-resistant Plasmodium falciparum malaria (29) because of their excellent efficacy (23, 31, 39, 41), their ability to slow or reverse the emergence of resistance (24, 30), and their ability to reduce malaria transmission (24, 30). Despite the coexistence of falciparum and vivax malaria in many parts of the world where malaria treatment is usually based on clinical diagnosis, there have been few studies assessing the efficacy of artemisinin derivatives in treating vivax malaria and none in the area of CQ-resistant P. vivax. Studies of artesunate (ART) monotherapy for P. vivax infection have demonstrated rapid clearance of fever and parasites (1, 6, 33, 43). However, with its very short half-life, no ART remains by the time of the first relapse of P. vivax infection, approximately 3 weeks after treatment begins, resulting in a high frequency of 28-day treatment failure with ART monotherapy (33). Even fewer data are available regarding the efficacy of ACT in treating vivax malaria. To address this deficit, the World Health Organization (WHO) has called for studies to assess the efficacy of ACT in the treatment of P. vivax, particularly in areas with CQ-resistant P. vivax (46). We recently found ART plus sulfadoxine-pyrimethamine (SP) to be highly effective (96%) in the treatment of falciparum malaria in Papua, Indonesia (39). As malaria is frequently diagnosed clinically without microscopic identification to the species level, the empirical use of ACT in settings such as Papua, where both species occur, would require that ACT be efficacious against both falciparum and vivax malaria.
P. vivax is intrinsically resistant to sulfadoxine and has acquired resistance to pyrimethamine in areas of Southeast Asia such as Thailand (16, 33). Triple mutations have been found in P. vivax dihydrofolate reductase (PvDHFR) at positions 57, 58, and 117. These mutations are associated with lower 48-h parasite reduction ratios following monotherapy of P. vivax infection with SP than are isolates with double mutations (at positions 58 and 117); however, no differences in cure rates have been demonstrated (16). Even when P. vivax is sensitive to pyrimethamine, SP therapy of vivax malaria results in slow clearance of parasites and fever (9). Therefore, SP is not recommended as monotherapy for P. vivax in Indonesia (13). However, when ART is combined with SP, the ART should rapidly reduce initial parasite biomass, with SP, a drug with a longer half-life, eradicating the small residual parasite load at a time of a persisting adequate concentration in blood (42). While this should allow for a shorter duration of ART than occurs when it is used as monotherapy (42), it requires that P. vivax demonstrate sensitivity to pyrimethamine; data supporting this have not been previously available from Papua.
Although the CQ-SP combination was later shown to have inadequate efficacy for falciparum malaria treatment in Papua Province (8, 38), it had been considered an option for the empirical management of malaria in Papua. We therefore undertook small pilot studies using modified WHO criteria to examine the efficacies of CQ and CQ-SP in vivax malaria treatment. We then evaluated the therapeutic efficacy of the ART-SP combination for the treatment of P. vivax malaria in an open study at Genyem Health Center, located in an area of Papua with CQ-resistant P. vivax (37).
We used P. vivax isolates from these combination studies to examine the relationship between mutations in the Pvdhfr gene and the therapeutic efficacies of SP-containing regimens for the treatment of vivax malaria.
MATERIALS AND METHODS
Study sites and subjects for efficacy studies.
Our open therapeutic efficacy studies were performed in 1999 at Genyem Health Center, Papua Province, an area where malaria with year-round transmission is meso-endemic (38, 39). The pilot CQ, CQ-SP, and ART-SP efficacy studies were performed with patients with vivax malaria who had been admitted to the center from February to April 1999. Following initial evidence for the efficacy of SP against P. vivax in this region of Papua, the efficacy study of ART-SP was extended to include a larger number of patients with vivax malaria, those admitted from August to November 1999.
In the absence of published guidelines for assessing the therapeutic efficacy of P. vivax treatment, patients with uncomplicated vivax malaria were selected on the basis of the draft 1997 WHO criteria for assessment of the therapeutic efficacy of antimalarial drugs for uncomplicated falciparum malaria in areas with low or moderate levels of transmission (44). A minimum of 500 parasites/μl was used as the cutoff for inclusion in the CQ and CQ-SP studies, and 1,000 parasites/μl was the minimum for the ART-SP study; these are more conservative levels than the 250-parasite/μl cutoff presently recommended in the 2002 WHO guidelines for the assessment of therapeutic efficacy in vivax malaria (45). The exclusion criteria were danger signs of severe and complicated malaria, pregnancy, lactation, and a history of drug hypersensitivity (44).
Informed consent was obtained from all adult study subjects and from a parent or guardian for minors. The study was approved by the Ethics Committee of the National Institute of Health Research and Development, the Indonesian Ministry of Health, Jakarta, Indonesia, and the Health Research Ethics Committee of the Menzies School of Health Research and Territory Health Services, Darwin, Australia.
Treatment.
In the pilot CQ and CQ-SP studies, treatment of patients with vivax malaria was randomized by day of attendance at the health center to consist of either CQ or CQ-SP. Treatment doses were CQ (Resochin; Bayer, Jakarta, Indonesia) at a base dose of 25 mg/kg of body weight (bw) administered orally over 3 days, alone or in combination with SP (Fansidar; Roche, New South Wales, Australia) (one tablet containing 500 mg of sulfadoxine and 25 mg of pyrimethamine) administered in a single dose on day 0 (based on 1.25 mg of pyrimethamine/kg of bw) (38). The open pilot study of the therapeutic response of ART-SP was undertaken with five vivax malaria patients and was then extended to include another 17 patients with symptomatic infections with P. vivax. Patients were treated with artesunate (Artesunat [Mekophar, Ho Chi Minh, Vietnam; 5 patients in the pilot phase] and Artesunate [Mediplantex, HaNoi, Vietnam; 17 patients in the extension phase]; one 50-mg tablet, 4 mg/kg of bw, administered in a single oral daily dose for 3 days) plus SP (Fansidar [Roche]; one tablet containing 500 mg of sulfadoxine and 25 mg of pyrimethamine, with a single oral dose given orally on day 0, based on a dose of pyrimethamine of 1.25 mg/kg of bw). The management of treatment failures was conducted according to Ministry of Health guidelines (13). Difficulties at the study site in Papua Province in 1999 prevented the enrollment of additional patients.
Clinical examination, staining of serial thick and thin films, and hemoglobin level measurements were performed on days 0, 1, 2, 3, 7, 14, and 28 (44). Giemsa-stained thick and thin films were read by a microscopist with 24 years of experience from the Indonesian National Reference Centre. Asexual- and sexual-stage parasites were counted for every 200 white blood cells, and parasitemia was calculated on the basis of a white blood cell count of 8,000/μl. A thick film was considered negative if no parasites were found in 100 high-power fields. Day 0 blood blots were collected on filter paper (39) and stored at 4°C until DNA extraction.
Assessment.
The primary end points were the therapeutic responses based on parasitological and clinical cure by days 14 and 28, determined by using the 1997 WHO protocol criteria (44).
Secondary end points in the ART-SP efficacy study were (i) fever clearance time, defined as the time (days) from initiation of treatment to fever clearance (i.e., patient remained afebrile based on reported history and had an axillary temperature of <37.5°C on day of follow-up); (ii) parasite clearance time, defined as the time (days) from the initiation of treatment to parasite clearance (i.e., parasite count fell below the level of microscopic detection); (iii) hematological recovery, defined as being cured on days 14 and 28, with a hemoglobin level greater than that on day 0; and (iv) tolerability and safety based on the incidence of adverse reactions. An adverse reaction was defined as the development of any symptom or sign that neither existed before the initiation of treatment nor was a classic symptom or sign of malaria or intercurrent infection.
Additional analyses.
Therapeutic outcome was also classified as treatment failure or adequate clinical and parasitological responses (ACPR) by using the 1997 WHO in vivo criteria for falciparum malaria (44). Treatment failure was defined as the development of one of the following during the follow-up period (failure in the first 3 days was considered early treatment failure, and failure from day 4 to day 28 was considered late treatment failure): danger signs or severe malaria in the presence of parasitemia with the same species as that on day 0, parasitemia on day 2 greater than that on day 0, parasitemia on day 3 greater than or equal to 25% of the day 0 parasitemia, the unscheduled return of the patient due to clinical deterioration in the presence of parasitemia, and the presence of parasitemia on any of the subsequent scheduled days of return (day 7, 14, or 28) with the same species as that on day 0. An ACPR was defined as the patient not developing any of the criteria of early or late treatment failure, with parasite clearance being confirmed throughout the follow-up period beyond day 3 (44).
Additional subjects included in the genotyping studies.
In addition to the subjects included in the Papuan Province efficacy studies described above, Pvdhfr genotyping studies were also performed with eight isolates of P. vivax from Radamata, Nusa Tenggara Timur (NTT), a province to the west of Papua, from patients treated with CQ in 1998 (all with an ACPR) (37). The sole additional available Papuan isolate was also genotyped; this isolate was from a patient with a mixed P. falciparum-P. vivax infection who had had late treatment failure of P. vivax following treatment in 1999 with SP (37).
Parasite DNA isolation, Pvdhfr amplification, and sequencing.
Genomic DNA was extracted by using Chelex-100 (17). Briefly, 40 μl of a 5% Chelex-100 solution, preheated to 80°C, was added to each sample. The samples were heated to 80°C for 10 min with intermittent vortex mixing. The samples were then centrifuged for 1 min, and the supernatant was transferred and kept at −20°C until used. Pvdhfr was amplified with primers PvDHFR1 (5′ ATGGAGGACCTTTCAGATGTATT 3′) and PvDHFR2 (5′ CCACCTTGCTGTAAACCAAAAAGTCCAGAG 3′). PCR was performed with 75 ng of each primer, 2.5 mM Mg2Cl, 0.2 mM each deoxynucleoside triphosphate (Promega, Madison, Wis.), 1.25 U of AmpliTaqGold (PE Applied Biosystems), and 2 μl of genomic DNA. The reaction mixture was initially heated at 94°C for 10 min, followed by 40 cycles at 94°C for 50 s, 55°C for 50 s, and 72°C for 50 s. The PCR product was purified with QIAquick spin columns (QIAGEN, Hilden, Germany) and sequenced directly from both directions. A mixed infection was reported when mixed sequence signals were observed at the specific nucleotide positions where mutations were observed in an otherwise clean DNA sequence.
Data analysis.
Data were entered and analyzed by using Epi-Info, version 6 (10). Differences in proportions with treatment failure were determined by using Fisher's exact test.
RESULTS
Therapeutic efficacy.
In pilot studies to assess whether SP added efficacy to treatment of P. vivax with CQ, 19 patients were randomized to treatment with either CQ (n = 9) (standard therapy for vivax malaria in Papua Province) or CQ-SP (n = 6), with each group having similar baseline characteristics (Table 1). Only one (11%) of those treated with CQ had an ACPR at 28 days (but without hematological recovery), with two having early treatment failure and six having late treatment failure (total treatment failure with CQ, 89%). In those treated with CQ-SP, four (67%) had ACPR at 28 days (50% with hematological recovery), with two (33%) having late treatment failure (results with CQ versus results with CQ-SP, P = 0.046 by a one-tailed Fisher exact test).
TABLE 1.
Enrollment characteristics of vivax malaria patients treated with CQ, CQ-SP, and ART-SP in Papua in 1999
| Characteristic | CQ (n = 9)a | CQ-SP (n = 6)b | ART-SP (n = 22) |
|---|---|---|---|
| Age (yr) (mean ± SD [range]) | 8.8 (1-27) | 4.3 (1-8) | 5.6 ± 6.9 (0.5-30) |
| Gender (% male) | 6 (67) | 4 (67) | 13 (59) |
| Ethnic group (% Papuan) | 78 | 100 | 77 |
| History of prior antimalarial use (%) | 33 | 50 | 59 |
| Axillary temp (°C) (range) | 37.1 (36-38.9) | 36.4 (36-36.7) | 37.1 (36.0-40.5) |
| Hemoglobin level (g/dl) (range) | 10.7 (6.3-17.7) | 8.9 (6.4-12.1) | 9.9 (6.6-17.6) |
| Asexual parasitemia (mean no. of parasites/μl) (range) | 8,756 (920-25,600) | 10,398 (440-38,000) | 3,951 (840-17,080) |
Includes three patients with mixed P. falciparum and P. vivax infection.
Includes two patients with mixed P. falciparum and P. vivax infection.
Based on this preliminary evidence for the efficacy of SP, 22 subjects were enrolled in the ART-SP efficacy study (Table 1). The patient ages and ethnic groups were similar for the pilot (n = 5) and extension (n = 17) phases of the study, with Papuan children comprising 80 and 94% of each phase, respectively. Three of the 22 patients enrolled in the ART-SP study were excluded: one withdrew consent on day 2, one developed P. falciparum infection on day 7, and the other moved from the study site on day 28 (he was included for analysis on day 14 but excluded from analysis on day 28). Thus, the ACPR rate was 100% (20 of 20) by day 14 and 89.5% (17 of 19) by day 28 (Table 2). No adverse reactions were identified.
TABLE 2.
Therapeutic efficacy of ART-SP in patients with uncomplicated malaria
| Endpointa | No. of patients (n = 22)b | % of patients (95% CI) | Clearance time (days) (mean ± SD [range]) |
|---|---|---|---|
| Primary | |||
| Day 14 ACPR | 20 | 100 (83.2-100) | |
| Day 28 ACPR | 17 | 89.5 (66.9-98.7) | |
| ETF | 0 | 0 | |
| Day 28 LTF | 2c | 10.5 (1.3-33.1) | |
| Secondary | |||
| FCT | 1.4 ± 0.6 (1-3) | ||
| PCT | 1.1 ± 0.2 (1-2) | ||
| Hb | 12 | 70.6 (44.0-89.7) |
ACPR, adequate clinical and parasitological response; ETF, early treatment failure; LTF, late treatment failure; FCT, fever clearance time; PCT, parasite clearance time; Hb, hematological response.
Three patients were excluded from evaluation on days 2, 7, and 28 for all end points except LTF.
Late treatment failure occurred on days 21 and 28.
Genetic mutations in Pvdhfr.
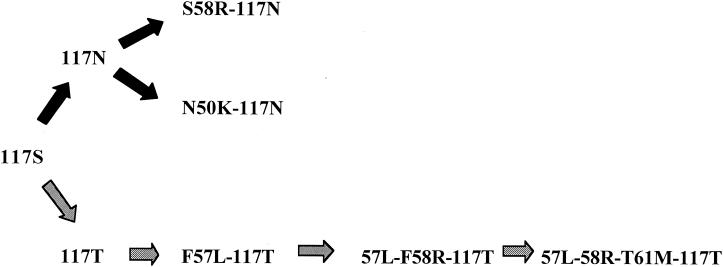
Pretreatment isolates were available from 31 vivax malaria patients from the Papuan efficacy studies. The Pvdhfr gene was amplified and sequenced from 26 patients. As two patients had mixed sequences of dhfr, 28 Pvdhfr genotypes were produced (Table 3). Of these 28 Papuan isolates, only 6 (21%) had wild-type amino acids of P33, N50, F57, S58, T61, S117, and I173. (Fig. 1 and Table 3). Double mutations were common (n = 13; 46%) and included combinations of R58 and N117 (n = 10), K50 and N117 (n = 2), and L57 and T117 (n = 1) (Fig. 1). Quadruple mutations in the gene encoding PvDHFR (L57, R58, M61, and T117) were found in 18% of the isolates (Table 4). Two-thirds of the samples had at least two mutations.
TABLE 3.
Frequency of pvdhfr-encoded mutations among clinical isolates of P. vivax in Papua
| Trial no. | Sequence polymorphisma at position:
|
No. of mutations | Therapeutic response (SP-containing regimen)b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 33 | 50 | 57 | 58 | 61 | 117 | 173 | |||
| G806 | P | N | F | S | T | S | I | 0 | ACPR (CQ-SP) |
| G903 | P | N | F | S | T | S | I | 0 | LTF (CQ-SP) |
| G104 | P | N | F | S | T | S | I | 0 | ACPR (CQ-SP) |
| G2060 | P | N | F | S | T | S | I | 0 | ACPR (ART-SP) |
| G2069 | P | N | F | S | T | S | I | 0 | ACPR (ART-SP) |
| G058 | P | N | F | S | T | S/T | I | Mixed (0 and 1) | CQ |
| G965 | P | N | F | S | T | N | I | 1 | ACPR (CQ-SP) |
| G359 | P | N | F | R | T | N | I | 2 | CQ |
| G457 | P | N | F | R | T | N | I | 2 | CQ |
| G535 | P | K | F | S | T | N | I | 2 | CQ |
| G126 | P | N | F | R | T | N | I | 2 | ACPR (CQ-SP) |
| G1040 | P | N | F | R | T | N | I | 2 | ACPR (ART-SP) |
| G743 | P | N | F | R | T | N | I | 2 | CQ |
| G2028 | P | N | F | R | T | N | I | 2 | ACPR (ART-SP) |
| G2041 | P | N | F | R | T | N | I | 2 | ACPR (ART-SP) |
| G2067 | P | N | F | R | T | N | I | 2 | ACPR (ART-SP) |
| G2090 | P | N | L | S | T | T | I | 2 | ACPR (ART-SP) |
| G2091 | P | K | F | S | T | N | I | 2 | ACPR (ART-SP) |
| G2099 | P | N | F | R | T | N | I | 2 | ACPR (ART-SP) |
| G2110 | P | N | F | R | T | N | I | 2 | ACPR (ART-SP) |
| G273 | P | N | L | R | T | T | I | 3 | CQ |
| G852 | P | N | L | R | M | T | I | 4 | LTF (SP) |
| G1015 | P | N | L | R | M | T | I | 4 | LTF (ART-SP) |
| G2042 | P | N | L | R | M | T | I | 4 | LTF (ART-SP) |
| G2095 | P | N | L | R | M | T | I | 4 | ACPR (ART-SP) |
| G2022 | P/S | N | F/L | R/S | M/T | T | I | Mixed (1 and 4) | ACPR (ART-SP) |
Wild-type amino acids are in roman-type, previously described polymorphisms are in italics, and novel polymorphisms are in bold type.
LTF, late treatment failure; CQ alone, treated only with CQ.
FIG. 1.
Stepwise drug selection processes in the mutations encoded by the Pvdhfr gene suggested by the observed allele structures in Papuan genotypes.
TABLE 4.
Relationship between the number of mutations in the Pvdhfr gene and clinical outcome following treatment of vivax malaria with an SP-containing regimen
| No. of mutations | No. (%) of isolatesa | No. (%) of isolates with ACPR with SP-containing regimen |
|---|---|---|
| 0 | 6 (21) | 4/5 (83) |
| 1 | 3 (11) | 2/2 (100) |
| 2 | 13 (46) | 9/9 (100) |
| 3 | 1 (4) | —b |
| 4 | 5 (18) | 2/5 (40) |
| Total | 28 |
Includes isolates from patients with mixed infections.
—, not treated with SP-containing regimen.
Of the 28 Papuan isolates genotyped, 21 were treated with an SP-containing regimen (5 with CQ-SP, 15 with ART-SP, and 1 with SP), with the remainder receiving CQ (Table 3). Treatment outcome was associated with the number of mutations (Table 4). Only 40% of those with the quadruple mutation had an ACPR to an SP-containing regimen compared with 94% of those with two or fewer mutations (P = 0.03; Fisher's exact test). The rapid parasiticidal effect of the ART component in those treated with ART-SP precluded meaningful analysis of the effect of the quadruple PvDHFR mutation on parasite clearance time. In all five cases with the quadruple mutation, the mutation at position 117 was S to T, whereas of the 13 isolates with the double mutation, only 1 had S117T (P = 0.0007; Fisher's exact test), with the remainder having an S-to-N mutation at this position.
Novel mutations were found at two residues. The first was a T-to-M mutation at position 61 (n = 5; 19%), which in each isolate was always linked to the triple mutation F57L-S58R-S117T, thereby forming a quadruple mutation (Table 3). The second novel mutation was an N50K mutation in two isolates, which in each case was linked to an S117N mutation. This was not associated with treatment failure; however, only one case was treated with an SP-containing regimen. One isolate had a P33S mutation, which did not result in treatment failure. Mutations at residue 173 were not detected in any of the isolates (Table 3). In contrast to the high incidence of multiple mutations in the PvDHFR in Papuan isolates, no mutations were found in any of the eight P. vivax isolates from NTT Province.
DISCUSSION
This is the first report concerning the efficacy of ACT for vivax malaria in a region with high rates of CQ-resistant P. vivax (46). Because of the very short half-life of ART, after 3 to 7 days of the treatment of symptomatic infection, no ART remains by the time of the first relapse, which in northeastern Papua occurs in 58% of subjects by day 30 (3, 4). A similarly high rate of early relapse is found in Thailand (33), and after clearance of the initial infection, the absence of circulating ART at the time of the first relapse is thought to account for the high (63%) rate of 28-day treatment failure found when ART is used alone (33). In a setting where the day 28 relapse rate was expected to be over 50%, we found a 90% day 28 cure rate with ART-SP. This suggests that the addition of SP to ART prevents or delays the appearance of the first relapse of P. vivax infection in Papua. It is consistent with the hypothesis that when ART is combined with SP, the ART rapidly reduces the initial parasite biomass, with SP, the drug with the longer half-life, eradicating or suppressing both the small residual parasite load and the emerging relapse parasite load at a time of a persisting adequate concentration in blood. The rapid initial fever and parasite clearances seen with ART-SP, similar to those previously found with ART monotherapy (1, 6, 33, 46), indicate that the addition of ART to SP overcomes the slow parasite and fever clearances ordinarily seen with SP monotherapy (9), a major reason underlying recommendations to avoid the use of SP monotherapy for P. vivax (13).
There is greater difficulty associated with efficacy studies with P. vivax than with P. falciparum, because of the difficulty in distinguishing recrudescences from relapses (4). The limitations of our studies in particular included small sample sizes, randomization of the CQ studies by day of attendance, and the presence of mixed infections. Furthermore, the slow parasite killing with SP may have only delayed rather than eradicated emerging parasitemia from either the recrudescence of initial infection or the first relapse. Current WHO efficacy protocols (45) fail to detect delayed treatment failure beyond day 28. However, the prevention of the expected relapses and the hematological recovery seen (32) do provide evidence for a therapeutic effect of SP against P. vivax in Papua. Although patient numbers were small, the preliminary finding of the improved efficacy of CQ-SP over CQ alone for vivax malaria provides further evidence for the modest efficacy of SP in Papua. While a study with larger numbers may confirm this, such a study would now be difficult to justify in Papua: the high rates of P. vivax and P. falciparum resistance to CQ (2, 3, 5, 21, 28, 38) and the poor efficacy of CQ-SP against P. falciparum in both northern (38) and southern (8) Papua render the combination of CQ-SP no longer a rational option for malaria treatment in this region.
Double (at positions 58 and 117) and quadruple (at positions 57, 58, 61, and 117) mutations in the Pvdhfr gene were common in the P. vivax isolates from Papua but were not found further west in NTT Province. Rates of CQ and SP resistance of P. falciparum are also higher in Papua Province than in the NTT province (38, 40; E. Tjitra, S. Suprianto, M. Dyer, B. J. Currie, and N. M. Anstey, abstract, Am. J. Trop. Med. Hyg. 61[Suppl.]:286-287, 1999), resulting in greater use of SP as a second line of therapy for P. falciparum infection in Papua and greater drug pressure on the dhfr gene of both species. Mass drug administration of CQ and pyrimethamine in the former Netherlands New Guinea in the late 1950s (15, 19), including the addition of pyrimethamine to salt (15, 20), may also have contributed to the high frequency of Pvdhfr mutations in Papua compared with that in NTT.
Mutations at four amino acid positions in PvDHFR, P33L, F57L, S58R, and S117N, have been reported previously (12, 16). Two types of PvDHFR alleles encoding either a double mutation of S57R-S117N or a triple mutation of F57L-S58R-S117N were observed in Thai and Indian samples. A single mutation of P33L was observed only in samples from Madagascar and Comoros islands (16). The PvDHFR double and triple mutant alleles have been linked to lower parasite reduction ratios (16). However, these studies have not found an association between Pvdhfr gene mutations and treatment failure (16). In the Papuan samples, we identified seven mutations in PvDHFR at six positions: P33S, N50K, F57L, S58R, T61M, and S117N/T, which correspond to amino acids 44, 51, 58, 59, 62, and 108 in PfDHFR, respectively. Among the seven mutations observed in PvDHFR, four have been reported in PfDHFR (N51I, C59R, and S108N/T) to result in resistance to pyrimethamine and cycloguanil (7, 14, 25, 26). The single S108N and S108T mutations result in increased resistance to pyrimethamine and cycloguanil, respectively. Combinations of S108N with the mutations N51I, C59R, and I164L were found to confer higher levels of resistance to pyrimethamine (7). Molecular modeling predicted that S108 is located at the active site in PfDHFR and that the S108N/T mutation results in displacement of pyrimethamine and cycloguanil from the binding site (11). Mutations at positions 51 and 59 were predicted to be distant from the active site but may affect the admission of the drug to the binding site (11). Since PvDHFR is highly homologous to PfDHFR in sequence, we would expect that it shares a high structure similarity with PfDHFR and that the mutations N50K, S58R, and S117N/T in PvDHFR have effects similar to those of the corresponding mutations in PfDHFR. Indeed, the Escherichia coli-expressed PvDHFR with the S58R and S117N mutations has been shown to confer resistance to both pyrimethamine and cycloguanil (18, 36). Mutations corresponding to P33S, F57L, and T61M have not been reported in PfDHFR. Recently, Leartsakulpanich et al. reported that E. coli-expressed PvDHFR with the F57L mutation confers a strong resistance to pyrimethamine and cycloguanil and that PvDHFR with the P33L mutation confers a small increase in resistance to pyrimethamine but not to cycloguanil (18). The effect of the T61M mutation requires further investigation.
Seven PvDHFR mutant alleles were identified from the Papuan samples that encoded the following mutations: S117N alone, S58R-S117N, N50K-S117N, S117T alone, F57L-S117T, F57L-S58R-S117T, and F57L-S58R-T61M-S117T. These observed allele structures strongly suggest that two different stepwise drug selection processes have occurred in the area: (i) S117N to S58R-S117N or N50K-S117N and (ii) S117T to F57L-S117T, then to F57L-F58R-S117T, and finally F57L-F58R-T61M-S117T (Fig. 1).
Since treatment failure with SP-containing regimens was significantly higher with the mutant allele of PvDHFR encoding F57L-S58R-T61M-S117T, it appears that the quadruple mutation confers higher resistance to SP than does the mutant allele encoding a double mutation (S58R-S117N or N50K-S117N). Although the sample number is small, the results indicate that the allele encoding a quadruple mutation may be used as a marker to predict clinical outcome following treatment with SP-containing regimens.
It is interesting that mutations of N50K and T61M have not been reported for PvDHFR and that the allele encoding the quadruple mutation F57L-S58R-T61M-S117T and the allele encoding the double mutation N50K-S117N have so far not been reported for other areas where malaria exists. One explanation may be that previous studies performed restriction fragment length polymorphism analysis for the presence or absence of the known mutations and did not perform sequencing, which may lead to an underestimation of the mutations in the samples. The second explanation may be that a different selection pressure was exerted in these areas. In P. falciparum, the S108N mutation is primarily associated with pyrimethamine resistance (7, 26), while S108T, combined with A16V, has been associated with cycloguanil resistance (25). S108T mutations have been found mainly in laboratory lines and are not commonly seen in field isolates (22, 34). It has been proposed that the S108T mutation may result in a significant loss of fitness to the mutant parasite, rendering mutant parasites less competitive than other parasites under the field conditions. In our set of P. vivax samples, S117T mostly occurred in tandem with F57L or with F57L-S58R-T61M. These additional mutations are likely to confer a higher level of resistance to pyrimethamine than S117T alone does and may compensate for the loss of fitness resulting from the S117T mutation.
In conclusion, treatment with ART-SP resulted in rapid clearance of parasites and fever and a high 28-day cure rate for vivax malaria in Papua, which is similar to that previously found with P. falciparum (39). At least two mutations in PvDHFR were found in the majority of Papua Province isolates with the newly described quadruple mutation associated with treatment failure. Rapid emergence of dhfr mutations is known to occur in P. falciparum in the face of continued use of antifolate monotherapy (27, 35) and is likely to also occur with P. vivax. The addition of ART to SP may delay the emergence of pyrimethamine-resistant P. vivax and SP-resistant P. falciparum. However, the continued use of SP in empirical monotherapy for clinical malaria in Papua since these studies were performed makes the progression of dhfr gene mutations likely in both species and threatens the future efficacy of this combination for both vivax and falciparum malaria.
Acknowledgments
This study received financial support from the Northern Territory Government Malaria-Tuberculosis Research Fellowships, The Nicholson-Hill Malaria Research Fund, and the Tudor Foundation.
We thank Bart Currie, Sumarjati Arjoso, Ferdinand Laihad, Budi Subianto, Tony Dimpudus, Krisman Hutadjulu, Ester Ayomi, Yusup, Gede Utomo, Neli, Sue Hutton, and the staff of the Regional, Provincial, District, and Subdistrict Health Offices, Papua, for support and both logistical and technical assistance. We also thank David Hipgrave and Bev Biggs for assistance with the delivery of ART.
REFERENCES
- 1.Alecrim, M., L. M. Carvalho, M. C. Fernandes, S. D. Andrade, A. C. Loureiro, A. R. Arcanjo, and W. D. Alecrim. 2000. Malaria treatment with artesunate (retocaps) in children of the Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 33:163-168. (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 2.Baird, J. K., H. Basri, Purnomo, M. J. Bangs, B. Subianto, L. C. Patchen, and S. L. Hoffman. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 44:547-552. [DOI] [PubMed] [Google Scholar]
- 3.Baird, J. K., H. Basri, B. Subianto, D. J. Fryauff, P. D. McElroy, B. Leksana, T. L. Richie, S. Masbar, F. S. Wignall, and S. L. Hoffman. 1995. Treatment of chloroquine-resistant Plasmodium vivax with chloroquine and primaquine or halofantrine. J. Infect. Dis. 171:1678-1682. [DOI] [PubMed] [Google Scholar]
- 4.Baird, J. K., B. Leksana, S. Masbar, D. J. Fryauff, M. A. Sutanihardja, Suradi, F. S. Wignall, and S. L. Hoffman. 1997. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am. J. Trop. Med. Hyg. 56:621-626. [DOI] [PubMed] [Google Scholar]
- 5.Baird, J. K., I. Wiady, D. J. Fryauff, A. Sutamihardja, B. Leksana, H. Widjaja, Kysdarmanto, and B. Subianto. 1997. In vivo resistance to chloroquine by Plasmodium vivax and Plasmodium falciparum at Nabire, Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 56:627-631. [DOI] [PubMed] [Google Scholar]
- 6.Batty, K. T., A. T. Le, K. F. Ilett, P. T. Nguyen, S. M. Powell, C. H. Nguyen, X. M. Truong, V. C. Vuong, V. T. Huynh, Q. B. Tran, V. M. Nguyen, and T. M. Davis. 1998. A pharmacokinetic and pharmacodynamic study of artesunate for vivax malaria. Am. J. Trop. Med. Hyg. 59:823-827. [DOI] [PubMed] [Google Scholar]
- 7.Cowman, A. F., M. J. Morry, B. A. Biggs, G. A. Cross, and S. J. Foote. 1988. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 85:9109-9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crouch-Chivers, P., Budiarto, and G. Waramori. 2001. Assessment of the therapeutic efficacy of sulfadoxine-pyrimethamine (SP) and SP plus chloroquine combination for uncomplicated Plasmodium falciparum malaria infections in Irian Jaya, Indonesia, p. 23-31. 2001 Annual Report, Public Health and Malaria Control Section. Public Health and Malaria Control Section, PT Freeport, Indonesia.
- 9.Darlow, B., H. Vrbova, S. Gibney, D. Jolley, J. Stace, and M. Alpers. 1982. Sulfadoxine-pyrimethamine for the treatment of acute malaria in children of Papua Province New Guinea. II. Plasmodium vivax. Am. J. Trop. Med. Hyg. 31:10-13. [DOI] [PubMed] [Google Scholar]
- 10.Dean, A. G., J. A. Dean, D. Coulombier, K. A. Brendel, C. D. Smith, A. H. Burton, R. C. Dicker, K. Sullivan, R. F. Fagan, and T. G. Arner.1995. Epi Info, version 6: a word-processing database and statistics program for public health on IBM-compatible microcomputers. Centers for Disease Control and Prevention, Atlanta, Ga.
- 11.Delfino, R. T., O. A. Santos-Filho, and J. D. Figueroa-Villar. 2002. Molecular modeling of wild-type and antifolate resistant mutant Plasmodium falciparum DHFR. Biophys. Chem. 98:287-300. [DOI] [PubMed] [Google Scholar]
- 12.de Pecoulas, P. E., R. Tahar, T. Ouatas, A. Mazabraud, and L. K. Basco. 1998. Sequence variations in the Plasmodium vivax dihydrofolate reductase-thymidylate synthase gene and their relationship with pyrimethamine resistance. Mol. Biochem. Parasitol. 92:265-273. [DOI] [PubMed] [Google Scholar]
- 13.Directorate General of Communicable Disease Control and Environmental Health. 1996. Malaria 3: pengobatan (treatment). Department of Health, Republic of Indonesia.
- 14.Foote, S. J., D. Galatis, and A. F. Cowman. 1990. Amino acids in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum involved in cycloguanil resistance differ from those involved in pyrimethamine resistance. Proc. Natl. Acad. Sci. USA 87:3014-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunawan, S. 1985. A review of the malaria situation in Irian Jaya. Bul. Penelit. Kesehat 13:1-13. [Google Scholar]
- 16.Imwong, M., S. Pukrittakayamee, S. Looareesuwan, G. Pasvol, J. Poirriez, N. J. White, and G. Snounou. 2001. Association of genetic mutations in Plasmodium vivax dhfr with resistance to sulfadoxine-pyrimethamine: geographical and clinical correlates. Antimicrob. Agents Chemother. 45:3122-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kain, K. C., R. A. Wirtz, I. Fernandez, E. D. Franke, M. H. Rodriguez, and D. E. Lanar. 1992. Serologic and genetic characterization of Plasmodium vivax from whole blood-impregnated filter paper discs. Am. J. Trop. Med. Hyg. 46:473-479. [DOI] [PubMed] [Google Scholar]
- 18.Leartsakulpanich, U., M. Imwong, S. Pukrittayakamee, N. J. White, G. Snounou, W. Sirawaraporn, and Y. Yuthavong. 2002. Molecular characterization of dihydrofolate reductase in relation to antifolate resistance in Plasmodium vivax. Mol. Biochem. Parasitol. 119:63-73. [DOI] [PubMed] [Google Scholar]
- 19.Metselaar, D. 1961. Seven years malaria research and residual house spraying in Netherlands New Guinea. Am. J. Trop. Med. Hyg. 10:327-334. [DOI] [PubMed] [Google Scholar]
- 20.Meuwissen, T. 1964. The use of medicated salt in an antimalaria campaign in West New Guinea. Trop. Geogr. Med. 16:245-255. [PubMed] [Google Scholar]
- 21.Murphy, G. S., H. Basri, Purnomo, E. M. Andersen, M. J. Bangs, D. L. Mount, J. Gorden, A. A. Lal, A. R. Purwokusumo, S. Harjosuwarno, et al. 1993. Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet 341:96-100. [DOI] [PubMed] [Google Scholar]
- 22.Nagesha, H. S., S. Din, G. J. Casey, A. I. Susanti, D. J. Fryauff, J. C. Reeder, and A. F. Cowman. 2001. Mutations in the pfmdr1, dhfr and dhps genes of Plasmodium falciparum are associated with in-vivo drug resistance in West Papua Province, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 95:43-49. [DOI] [PubMed] [Google Scholar]
- 23.Nosten, F., C. Luxemburger, F. O. ter Kuile, C. Woodrow, J. Pa Eh, T. Chongsuphajaisiddhi, and N. J. White. 1994. Treatment of multidrug resistant Plasmodium falciparum malaria with a 3-day artesunate mefloquine combination. J. Infect. Dis. 170:971-977. [DOI] [PubMed] [Google Scholar]
- 24.Nosten, F., M. van Vugt, R. Price, C. Luxemburger, K. L. Thway, A. Brockman, R. McGready, F. ter Kuile, S. Looareesuwan, and N. J. White. 2000. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet 356:297-302. [DOI] [PubMed] [Google Scholar]
- 25.Peterson, D. S., W. K. Milhous, and T. E. Wellems. 1990. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA 87:3018-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson, D. S., D. Walliker, and T. E. Wellems. 1988. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc. Natl. Acad. Sci. USA 85:9114-9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plowe, C. V., J. F. Cortese, A. Djimde, O. C. Nwanyanwu, W. M. Watkins, P. A. Winstanley, J. G. Estrada-Franco, R. E. Mollinedo, J. C. Avila, J. L. Cespedes, D. Carter, and O. K. Doumbo. 1997. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J. Infect. Dis. 176:1590-1596. [DOI] [PubMed] [Google Scholar]
- 28.Pribadi, W., I. Sutanto, S. Atmosoedjono, R. Rasidi, L. K. Surya, and L. Susanto. 1998. Malaria situation in several villages around Timika, south central Irian Jaya, Indonesia. Southeast Asian J. Trop. Med. Public Health 29:228-235. [PubMed] [Google Scholar]
- 29.Price, R. N. 2000. Artemisinin drugs: novel antimalarial agents. Exp. Opin. Investig. Drugs 9:1815-1827. [DOI] [PubMed] [Google Scholar]
- 30.Price, R. N., F. Nosten, C. Luxemburger, F. O. ter Kuile, L. Paiphun, T. Chongsuphajaisiddhi, and N. J. White. 1996. Effects of artemisinin derivatives on malaria transmissibility. Lancet 347:1654-1658. [DOI] [PubMed] [Google Scholar]
- 31.Price, R. N., F. Nosten, C. Luxemburger, M. van Vugt, L. Phaipun, T. Chongsuphajaisiddhi, and N. J. White. 1997. Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 91:574-577. [DOI] [PubMed] [Google Scholar]
- 32.Price, R. N., J. A. Simpson, F. Nosten, C. Luxemburger, L. Hkirjaroen, F. ter Kuile, T. Chongsuphajaisiddhi, and N. J. White. 2001. Factors contributing to anemia after uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 65:614-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pukrittayakamee, S., A. Chantra, J. A. Simpson, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeder, J. C., K. H. Rieckmann, B. Genton, K. Lorry, B. Wines, and A. F. Cowman. 1996. Point mutations in the dihydrofolate reductase and dihydropteroate synthetase genes and in vitro susceptibility to pyrimethamine and cycloguanil of Plasmodium falciparum isolates from Papua Province New Guinea. Am. J. Trop. Med. Hyg. 55:209-213. [DOI] [PubMed] [Google Scholar]
- 35.Sibley, C. H., J. E. Hyde, P. F. Sims, C. V. Plowe, J. G. Kublin, E. K. Mberu, A. F. Cowman, P. A. Winstanley, W. M. Watkins, and A. M. Nzila. 2001. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 17:582-588. [DOI] [PubMed] [Google Scholar]
- 36.Tahar, R., P. E. de Pecoulas, L. K. Basco, M. Chiadmi, and A. Mazabraud. 2001. Kinetic properties of dihydrofolate reductase from wild-type and mutant Plasmodium vivax expressed in Escherichia coli. Mol. Biochem. Parasitol. 113:241-249. [DOI] [PubMed] [Google Scholar]
- 37.Tjitra, E. 2001. Improving the diagnosis and treatment of malaria in eastern Indonesia. Ph.D. thesis. Northern Territory University, Darwin, Australia.
- 38.Tjitra, E., S. Suprianto, and N. M. Anstey. 2002. Higher gametocyte prevalence following failure of treatment of Plasmodium falciparum malaria with sulfadoxine-pyrimethamine and the combination of chloroquine plus sulfadoxine-pyrimethamine: implications for progression of anti-folate resistance. Trans. R. Soc. Trop. Med. Hyg. 96:434-437. [DOI] [PubMed] [Google Scholar]
- 39.Tjitra, E., S. Suprianto, B. J. Currie, P. S. Morris, J. R. Saunders, and N. M. Anstey. 2001. Therapy of uncomplicated falciparum malaria: a randomized trial comparing artesunate plus sulfadoxine-pyrimethamine versus sulfadoxine-pyrimethamine alone in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 65:309-317. [DOI] [PubMed] [Google Scholar]
- 40.Tjitra, E., S. Suprianto, M. E. Dyer, B. J. Currie, and N. M. Anstey. 2001. Detection of histidine rich protein 2 and panmalarial ICT Malaria Pf/Pv test antigens after chloroquine treatment of uncomplicated falciparum malaria does not reliably predict treatment outcome in eastern Indonesia. Am. J. Trop. Med. Hyg. 65:593-598. [DOI] [PubMed] [Google Scholar]
- 41.von Seidlein, L., P. Milligan, M. Pinder, K. Bojang, C. Anyalebechi, R. Gosling, R. Coleman, J. I. Ude, A. Sadiq, M. Duraisingh, D. Warhurst, A. Alloueche, G. Targett, K. McAdam, B. Greenwood, G. Walraven, P. Olliaro, and T. Doherty. 2000. Efficacy of artesunate plus pyrimethamine-sulphadoxine for uncomplicated malaria in Gambian children: a double-blind, randomised, controlled trial. Lancet 355:352-357. [DOI] [PubMed] [Google Scholar]
- 42.White, N. J. 1997. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 41:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilairatana, P., U. Silachamroon, S. Krudsood, P. Singhasivanon, S. Treeprasertsuk, V. Bussaratid, W. Phumratanaprapin, S. Srivilirit, and S. Looareesuwan. 1999. Efficacy of primaquine regimens for primaquine-resistant Plasmodium vivax malaria in Thailand. Am. J. Trop. Med. Hyg. 61:973-977. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 1997. Assessment of therapeutic efficacy of antimalarial drugs for uncomplicated falciparum malaria, draft. 28.2.97 ed. Division of Control of Tropical Diseases, World Health Organization, Geneva, Switzerland.
- 45.World Health Organization. 2002. Monitoring antimalarial drug resistance: report of a WHO consultation. World Health Organization, Geneva, Switzerland.
- 46.World Health Organization. 1998. The use of artemisinin & its derivatives as anti-malarial drugs. Malaria Unit, Division of Control of Tropical Disease, World Health Organization, Geneva, Switzerland.