Abstract
The postantifungal effect (PAFE) of amphotericin B was determined with a BacT/Alert automated system, which is based on the colorimetric detection of CO2. The levels of accuracy and precision of the automated method were high. Longer PAFEs were obtained for Candida albicans (P < 0.001), C. glabrata (P < 0.01), and C. krusei (P < 0.01) at increasing amphotericin B concentrations and exposure times.
The postantifungal effect (PAFE) has most commonly been determined by monitoring regrowth with viable counts at certain intervals after removal of the drug (5, 6, 9). The aim of the present investigation was to develop and validate a new, less laborious and time-consuming automated method for determination of PAFE, based on the differences in time required for the exposed cultures and controls to reach a critical value of CO2 production. Using this method, we studied the effects of various concentrations and exposure times of amphotericin B on the PAFE against different Candida species.
Candida albicans ATCC 90028, C. krusei ATCC 6258, and C. glabrata ATCC 90030 were used. Amphotericin B powder (Fungizone; Bristol-Myers Squibb, Bromma, Sweden) was dissolved in sterile water as a stock solution of 5 mg/ml and stored at −70°C until required. All tests were performed using RPMI 1640 medium (Sigma) supplemented with 0.5% dextrose and l-glutamine and buffered to a pH of 7.0 with morpholinepropanesulfonic acid (MOPS). The MIC of amphotericin B, as determined by the broth macrodilution method using National Committee for Clinical Laboratory Standards guideline M27-A (7), was 0.5 μg/ml for all three strains.
Time-kill curve procedure.
Yeast cells were suspended in 15 ml of RPMI medium and were incubated on a shaker at 37°C for 6 to 8 h. A 150-μl aliquot from this culture was transferred into 15 ml of fresh RPMI 1640 medium and was incubated as described above for 16 to 17 h. Thereafter, the cell concentration was adjusted spectrophotometrically to approximately 106 CFU/ml. A total of 10 ml of the yeast suspension was then added to either 10 ml of RPMI medium alone (control) or amphotericin B diluted in RPMI to final concentrations of 1, 2.5, 5, and 10 times the MIC. The solutions were placed on the shaker and agitated at 37°C. At predetermined time points (0, 0.5, 1, 2, 4, 6, 8, and 24 h), 0.1-ml samples were removed from each test suspension and serially diluted and 0.1-ml aliquots were spread in duplicate on Sabouraud dextrose agar plates and incubated at 35°C overnight. The experiments were done on three separate occasions for C. albicans and once for C. krusei and C. glabrata. Time-kill curves of averaged colony counts (log10 CFU/ml) versus time (h) were constructed.
Determination of PAFE.
Yeast cells were cultured as described above. The cell concentration was adjusted to 106 CFU/ml. Aliquots (10 ml) of this yeast suspension were added to either 10 ml of RPMI medium alone (control) or amphotericin B diluted in RPMI to final concentrations of 1, 2.5, 5, 10, and 20 times the MIC. Viable counts of each test solution were made by plating 0.1 ml of 10−3 dilutions on Sabouraud plates in duplicate. Test cultures were then placed on the shaker and agitated for 30 or 60 min at 37°C. Amphotericin B was then removed by filtering the cultures through a 0.45-μm-pore-diameter membrane filter (Sartorius, Goettingen, Germany). The filters were washed with 100 ml of phosphate-buffered saline, transferred into tubes with 20 ml of fresh phosphate-buffered saline, and vortexed gently to dislodge yeast cells from the filters. The control cultures were treated in the same way. Three to four different dilutions of the control were made to obtain a control with a concentration of inoculum as close as possible to the concentrations of inocula present in the cultures exposed to amphotericin B. Viable counts of exposed and control cultures were performed as described above. Aliquots (2 ml) of each culture were injected into vented Pedi-BacT aerobic pediatric culture bottles (gift from Organon Teknika Corp., Göteborg, Sweden). Triplicate Pedi-BacT bottles for every amphotericin B-exposed culture and control were used on each occasion. The bottles were loaded into the BacT/Alert microbial detection system and were incubated at 36°C until a positive signal was obtained. The duration of PAFE was calculated by using the formula PAFE = T − C (2), where T is the time required for the growing amphotericin B-exposed yeast culture to produce CO2 and change the color of the gas-permeable sensor in the bottom of the bottle from blue-green to yellow and the BacT/Alert instrument to produce a positive signal (8) and C is the time required for the control bottles to produce a positive signal. C. albicans experiments were repeated on four separate occasions, and C. krusei and C. glabrata experiments were repeated on two occasions.
Growth curves of C. albicans ATCC 90028 with three different starting inocula.
Yeast cells were cultured as described above. The cell concentration was adjusted to 5 × 105 CFU/ml. Aliquots (10, 5, and 1 ml) of this yeast suspension were transferred to RPMI-dextrose medium in Falcon tubes for a final culture volume of 20 ml. Cultures were placed on the shaker and agitated at 37°C. At predetermined time points (0, 2, 4, 6, 8, and 10 h), 0.1-ml samples were removed, diluted, and cultured as described for the time-kill procedure. The experiments were done four times. Growth curves of averaged colony counts (log10 CFU/ml) versus time (h) were constructed.
Statistical analysis.
Analysis of variance was used to analyze differences in the growth rates of different inocula and to compare the PAFEs of various concentrations and areas under the curves (AUCs). Regression analysis was employed to determine whether there were linear relationships between the time required for a positive signal and the inoculum size and between the PAFE and the concentration of amphotericin B. The method error (s) used in the precision analysis was estimated on the basis of duplicates by using the formula s = √∑d2/2n, where d is the difference in growth time between the duplicates and n is the number of duplicate pairs. The precision was calculated as the method error (in percent) of the mean of the each determination. Values are expressed as means ± standard error. STATISTICA software (StatSoft, Inc., Tulsa, Okla.) was used in the analysis of variance and regression analyses.
Time-kill curves showed that amphotericin B exhibited marked concentration-dependent killing activity against all three Candida species. A reduction of ≥3 log10 in C. albicans CFU/ml from that of the starting inoculum was noted in the first 2 h after exposure to 10 times the MIC and at 4 h after exposure to 5 and 2.5 times the MIC. A similar reduction was observed for C. krusei after 4 h of exposure to 10 and 5 times the MIC and for C. glabrata after 6 h of exposure to the same concentrations. Exposure to 2.5 times the MIC reduced the colony counts of C. krusei and C. glabrata ≥3 log10 in 6 and 8 h, respectively.
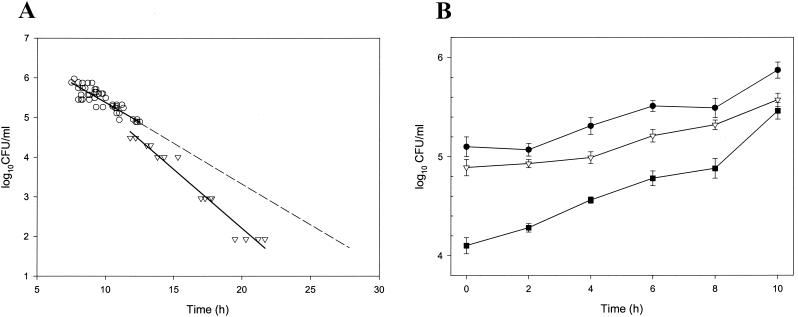
The times to a positive growth signal of the unexposed controls of C. albicans ATCC 90028 were analyzed in relation to the viable counts made before the yeast cells were injected into the Pedi-BacT bottles. Within the range of 104.3 to 106.0 CFU/ml, the time to a positive signal correlated strongly (with a limited deviation) to the initial numbers of CFU/ml, as measured by viable counts (r = −0.89, n = 62). The precision of the automated method was 2.3%. The largest deviation from the regression line was seen with the inocula of the smallest sizes (104.5 and 104.3 CFU/ml), suggesting a tendency of faster growth. Therefore, the growth rates of additional samples (≤104.0 CFU/ml) were tested and it was demonstrated that all starting inocula <104.5 CFU/ml in size had faster growth rates than larger inocula (Fig. 1A). To exclude the possibility that this phenomenon was due to the method, growth curves of C. albicans ATCC 90028 with starting inocula within the range anticipated in the PAFE experiments were constructed (Fig. 1B). The growth rate of the culture with the smallest starting inoculum amount was significantly higher than that of the cultures with larger inoculum amounts (P < 0.01). The precision of the viable count determinations was 5.0%.
FIG. 1.
(A) Relationship between log10 CFU/ml of the starting inocula and time to positive growth signal in the control Pedi-BacT bottles. The upper line (○) represents a regression line where y = 7.36 − 0.24x (for inocula of sizes greater than 4.5 log10 CFU/ml [n = 56]). The lower line (▿) represents a regression line where y = 8.15 − 0.30x (calculated from inocula of sizes <4.5 log10 CFU/ml [n = 17]). The regression line representing inocula of larger sizes (broken line) has been extended to allow comparison with that representing those of smaller size (lower solid line). Some of the symbols represent multiple samples with identical values. (B) Growth curves of C. albicans ATCC 90028 with three different starting inoculum concentrations.
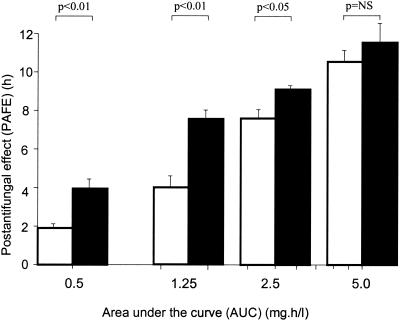
With increasing drug concentrations, there was a highly significant increase in the PAFE (P < 0.001) on C. albicans ATCC 90028 after both 30 and 60 min of exposure to amphotericin B (Table 1). The relationship between the PAFE and the logarithm of the concentration was close to linear for both exposure times (r30 min = 0.97; r60 min = 0.99). The combined effects of different concentrations and durations of exposure time (expressed as AUCs) are shown in Fig. 2. An exposure time of 60 min generated longer-lasting PAFEs than that of 30 min at the same AUC. There was a strong correlation between the killing rate of Candida cells during exposure to amphotericin B and the corresponding PAFE (r = 0.81; P < 0.001).
TABLE 1.
PAFE of amphotericin B against three Candida species
| Amphotericin B concn (μg/ml) | PAFE (h) (mean ± SE)
|
||
|---|---|---|---|
| C. albicans ATCC 90028 | C. krusei ATCC 6258 | C. glabrata ATCC 90030 | |
| 30-min exposure | |||
| 0.5 | 0.96 ± 0.18 | NDa | ND |
| 1.25 | 2.54 ± 0.13 | ND | ND |
| 2.5 | 3.92 ± 0.29 | 3.33 ± 0.27 | 3.19 ± 0.75 |
| 5.0 | 7.67 ± 0.42 | ND | ND |
| 10 | 10.67 ± 1.01 | 9.65 ± 0.05 | 5.02 ± 1.44 |
| 60-min exposure | |||
| 0.5 | 4.04 ± 0.42 | ND | ND |
| 1.25 | 7.68 ± 0.40 | 5.27 ± 0.40 | 4.18 ± 0.58 |
| 2.5 | 9.13 ± 0.15 | ND | ND |
| 5.0 | 11.54 ± 0.54 | 14.24 ± 0.20 | 6.65 ± 0.65 |
ND, not done.
FIG. 2.
The relationships between AUCs of amphotericin B at various concentrations and PAFEs on C. albicans ATCC 90028 after exposure times of 30 (□) and 60 (▪) min.
Some variation in the duration of the PAFE for the C. glabrata and C. krusei strains in comparison with that for C. albicans was noted (Table 1). However, an increase in PAFE at higher concentrations was also observed for C. krusei and C. glabrata (P < 0.01). The PAFE generated by 60 min of exposure was significantly longer lasting than that generated by 30 min of exposure at an AUC of 1.25 mg · h/liter (P < 0.05). At an AUC of 5 mg · h/liter there were similar findings, although they were significant for the C. krusei strain only (P < 0.05).
The accuracy of the present method was shown to be very good, since there was a linear relationship between the time to a positive signal and the log10 CFU of the starting inoculum and the deviation from the regression line was in the range that can be expected from the precision of the viable count determinations. When automated methods for the determination of PAFE are used, it is very important that the inoculum size of the diluted control and the inoculum sizes of the antifungal-exposed cultures are the same. Based on the precision of the viable count method and an inoculum size around 105 CFU/ml, the maximum acceptable difference in the size of the starting inocula (injected into the Pedi-BacT bottles) of the amphotericin B-exposed culture and that of the diluted control was set to <0.4 log10 CFU/ml. Variations in inoculum size accounted for less than 5% of the differences in times to a positive signal. Another automated method (based on turbidimetric measurement of fungal growth) has recently been used for PAFE determinations (3). Unfortunately, the methods cannot be compared, since no data on accuracy and precision were presented in that study.
In determining fungal growth, the method error of the BacT/Alert system was lower than that of the viable count system. In strains with faster growth rates at low inoculum sizes, the traditional methods might lead to underestimation of the PAFE unless the control were diluted, which would make these methods more laborious, and so the automated method presented here may offer an advantage.
Using the automated in vitro method, it was possible to demonstrate that the PAFE of amphotericin B was concentration and time dependent, as has previously been shown (9), and that the PAFE was proportional to the log10 concentration of amphotericin B. At an exposure time of 0.5 h, prior to the onset of killing, the PAFE could be demonstrated but was lower than that caused with a similar AUC at an 1-h exposure time. Though the clinical importance of this finding is yet uncertain, the concentration-dependent PAFE and killing rate indicate that for a given fungicidal effect a smaller total amount of amphotericin B may be required if higher doses are administered more infrequently, as was earlier proposed by Bindschadler and Bennett (1), and also that the recently proposed regimen of the prolongation to 24 h of amphotericin B infusion (4) may be associated with a reduction in effect. However, this hypothesis needs further experimental and clinical investigation.
REFERENCES
- 1.Bindschadler, D. D., and J. E. Bennett. 1968. A pharmacologic guide to the clinical use of amphotericin B. J. Infect. Dis. 120:427-436. [DOI] [PubMed] [Google Scholar]
- 2.Craig, W. A., and S. Gudmundsson. 1996. The postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, Md.
- 3.Ellepola, A. N., and L. P. Samaranayake. 1998. The postantifungal effect (PAFE) of antimycotics on oral C. albicans isolates and its impact on candidal adhesion. Oral Dis. 4:260-267. [DOI] [PubMed] [Google Scholar]
- 4.Ericsson, U., B. Seifert, and A. Schaffner. 2001. Comparison of effects of amphotericin B deoxycholate infused over 4 or 24 hours: randomised controlled trial. Br. Med. J. 322:579-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klepser, M. E., E. J. Wolfe, R. N. Jones, C. H. Nightingale, and A. Pfaller. 1997. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 41:1392-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minguez, F., J. E. Lima, M. T. Garcia, and J. Prieto. 1996. Influence of human serum on the postantifungal effect of four antifungal agents on Candida albicans. Chemotherapy 42:273-279. [DOI] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Thorpe, T. C., M. L. Wilson, J. E. Turner, J. L. DiGuiseppi, M. Willert, S. Mirrett, and L. Reller Barth. 1990. BacT/Alert: an automated colorimetric microbial detection system. J. Clin. Microbiol. 28:1608-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnidge, J. D., S. Gudmundsson, B. Vogelman, and W. A. Craig. 1994. The postantibiotic effect of antifungal agents against common pathogenic yeasts. J. Antimicrob. Chemother. 34:83-92. [DOI] [PubMed] [Google Scholar]