Abstract
The intracellular penetration and activity of linezolid in human polymorphonuclear leukocytes and tissue-cultured cells (McCoy) were evaluated. Linezolid reached intracellular concentrations slightly greater than extracellular ones in both types of cell. The uptake was rapid and not saturable and was affected by environmental temperature and cell viability. Linezolid showed slight intracellular activity against Staphylococcus epidermidis at high extracellular concentrations.
The penetration and intracellular activity of antimicrobial agents in phagocytes are particularly important under conditions in which the infecting microorganism is able to survive and multiply. Several infecting bacteria, however, are able to multiply within nonphagocytic cells, particularly epithelial cells. For these infections, the use of agents capable of accumulating and remaining active in such cells could be of potential clinical interest (9). The high intracellular accumulation of an antimicrobial agent does not always mean good intracellular activity. It is important, then, to evaluate whether intracellular antimicrobial agents remain active against bacteria. Linezolid is an oral oxazolidinone antibacterial agent that acts by inhibiting bacterial protein synthesis (4, 13-15) and has a wide spectrum of activity against gram-positive organisms including methicillin-resistant Staphylococcus aureus, penicillin- and cephalosporin-resistant pneumococci, and vancomycin-resistant Enterococcus faecalis and Enterococcus faecium, as well as anaerobes such as Clostridium spp., Peptostreptococcus spp., and Prevotella spp. (1, 5-7). Linezolid is bacteriostatic against most susceptible organisms, but displays bactericidal activity against some strains of pneumococci, Bacteroides fragilis, and Clostridium perfringens. (2, 3). There is no information about the intracellular pharmacology of these compounds in human phagocytes and nonphagocytic cells. The purpose of this study is to evaluate the uptake of linezolid by phagocytes (polymorphonuclear leukocytes [PMNs]) and nonphagocytic cells (McCoy). The intracellular activities of this compound against Staphylococcus aureus and Staphylococcus epidermidis were also evaluated.
The uptake of radiolabeled linezolid (167 μCi/mg; Pharmacia.) by phagocytes and nonphagocytic cells was determined by a velocity gradient centrifugation technique, as described by Klempner and Styrt (8). In these experiments, phagocytes or tissue-cultured cells were incubated in Hanks' balanced salt solution containing different concentrations of linezolid (1 to 40 μg/ml). After different periods of incubation at 37°C, the cells were separated from the extracellular solution by centrifugation through a water-impermeable silicone oil barrier in a microcentrifuge tube. A 10-μl aliquot of the extracellular medium and the entire cell pellet, obtained by cutting off the portion of the microcentrifuge tube containing the pellet, were placed in 3 ml of scintillation fluid (Ready Micro; Beckman Instruments, Inc., Fullerton, Calif.) and counted with a liquid scintillation counter (model LS 1801; Beckman). The intracellular water space was measured by using tritiated water and the extracellular marker [14C]polyethylene glycol (1.4 mCi/g; Amersham International, Plc., Buckinghamshire, United Kingdom). The cells were incubated with these radiolabeled compounds for 2 min at 37°C, and then the cells were separated from extracellular fluid by velocity gradient centrifugation and counted with a liquid scintillation counter. The total water content of the cell pellet was corrected for trapped extracellular water (i.e., polyethylene glycol space) to obtain the intracellular water space. The accumulation rate of the antimicrobial agent in PMN (cell-associated drug) or tissue-cultured cells was calculated and expressed as a cellular/extracellular concentration (C/E) ratio (11). The data are expressed as means ± standard deviations. Differences between groups were compared by variance analysis, used to assess statistical significance at P ≤ 0.05.
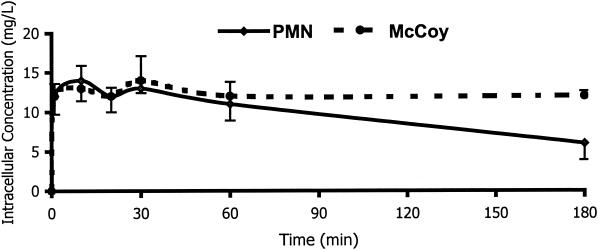
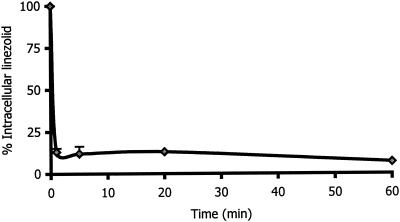
Figure 1 shows the kinetics of the uptake of linezolid by human PMNs and McCoy cells. The intracellular penetration of linezolid was rapid, reaching intracellular concentrations 1.2 times higher than the extracellular concentrations after 20 min of incubation in both kinds of cells. After 180 min of incubation, the C/E ratio values remained the same for McCoy cells, but had decreased by around 50% in PMNs. To evaluate whether linezolid that had been taken up by human PMNs was tightly bound to cellular components, we evaluated the kinetics of efflux (Fig. 2). The elution of linezolid from human PMN was rapid. After 10 min of incubation in an antimicrobial-free medium, 90% of linezolid was released. The intracellular penetration of linezolid into PMN and McCoy cells was not saturable at extracellular concentrations ranging from 1 to 40 mg/liter. The C/E ratios in PMNs ranged from 0.9 (extracellular concentration, 40 mg/liter) to 1.3 (extracellular concentration, 20 mg/liter). At the same extracellular concentration, the C/E ratios ranged from 1.1 (extracellular concentration, 40 mg/liter) to 1.3 (extracellular concentrations, 1, 5, and 20 mg/liter) in McCoy cells.
FIG. 1.
Kinetics of linezolid uptake by human PMNs and McCoy cells (n = 4). Experiments were carried out at extracellular concentrations of 10 mg/liter at 37°C. Data are expressed as means ± standard deviations.
FIG. 2.
Efflux of linezolid from human PMNs (n = 3). After incubation with 10 mg of linezolid per liter for 20 min, the cells were washed and resuspended in antimicrobial agent-free medium. Cell-associated linezolid was then measured at different times.
Further studies to elucidate the mechanisms for linezolid uptake by PMNs were performed as described previously (10). The influence of environmental temperature (4 versus 37°C), cell viability, and pH (5 to 8) and the effects of human serum, pooled serum (5 and 10%), and metabolic inhibitors (1.5 × 10−3 M [each] sodium fluoride and sodium cyanide; Sigma Chemical Co., St. Louis, Mo.) on the uptake of linezolid by PMNs were evaluated. The intracellular penetration of linezolid in PMNs was also measured after stimulating the cells with 200 nM phorbol myristate acetate (PMA; Sigma) and after phagocytosis of opsonized staphylococci (in 5% pooled human serum at a 10/1 ratio of bacteria to PMNs) and opsonized zymosan (0.9 mg/liter; Sigma) (Table 1). Cell-associated linezolid decreased in viable cells at 4°C (C/E ratio, 0.75 ± 0.15; control, 1.2 ± 0.4) and increased significantly (P < 0.05) when dead cells at 37°C were used (C/E ratio, 1.98 ± 0.15). A possible explanation for this increase might be that the formalin used to kill PMNs caused structural changes in the PMNs, which favored nonspecific binding of the linezolid. This hypothesis is currently under investigation. The uptake of this new oxazolidinone was not affected by external pH (which ranged from 5 to 8). Concentrations of pooled human serum and different metabolic inhibitors (NaCN and NaF) did not affect the intracellular penetration of this antimicrobial agent. Neither stimulation of the PMNs by a membrane activator (PMA) nor phagocytosis of opsonized S. aureus and zymosan significantly affected the intracellular penetration of linezolid. Most data indicated that a possible passive mechanism was involved in the intracellular penetration of this agent (7, 12).
TABLE 1.
Effects of various conditions on intracellular penetration of linezolid in human PMNsa
| Condition | C/E ratiob |
|---|---|
| Control (viable cells, 37°C, pH 72) | 1.20 ± 0.40 |
| Viable cells, 4°C | 0.75 ± 0.17c |
| Dead cells, 37°C | 1.98 ± 0.15c |
| pH | |
| 5 | 1.30 ± 0.42 |
| 6 | 1.13 ± 0.171 |
| 7 | 1.18 ± 0.39 |
| 8 | 1.20 ± 0.14 |
| Sodium cyanide | 1.25 ± 0.21 |
| Sodium fluoride | 1.20 ± 0.14 |
| Human serum | |
| 5% | 1.37 ± 0.12 |
| 10% | 1.47 ± 0.14 |
| Opsonized PMA | 1.25 ± 0.21 |
| Opsonized S. aureus | 1.35 ± 0.13 |
| Zymosan | 1.3 ± 0.27 |
The conditions examined include environmental temperature, cell viability, external pH, metabolic inhibitors, serum, membrane activation, and phagocytosis of S. aureus and zymosan.
Experiments (n = 4) were carried out for 20 min at extracellular concentrations of 10 mg/liter.
P < 0.05 compared with the control.
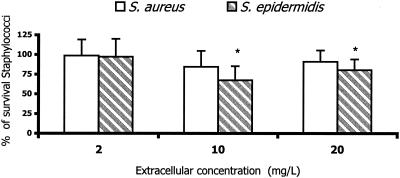
To evaluate the intracellular activities of antimicrobial agents, a previously described method was used (11). S. aureus ATCC 25923 and S. epidermidis ATCC 41134 were used for killing assays. Susceptibility studies were determined by microdilution assay. The MICs of linezolid for these strains were 2 and 1 mg/liter, respectively. The data were expressed as percentages of surviving staphylococci compared with the control levels (without antimicrobial agents) at 3 h. In addition to determining bacterial survival, morphological studies were also routinely performed, at time zero and after 3 h of incubation, to evaluate the disposition of bacteria (cell associated or extracellular). All assays were performed in duplicate with PMNs from five different donors. The data are expressed as means ± standard deviations. Differences between groups were compared by variance analysis, which was used to assess statistical significance at P ≤ 0.05. At extracellular concentrations attainable in human serum, linezolid did not affect the intracellular survival of staphylococci. At high extracellular concentrations (10 and 20 mg/liter), linezolid showed slight intracellular activity against S. epidermidis, but not against S. aureus (Fig. 3). The differences in the intracellular activity might be related to differences in the intrinsic activity of this compound against both strains.
FIG. 3.
Activity of linezolid against intracellular S. aureus and S. epidermidis in human PMNs (n = 4). Data are expressed as percentages of surviving bacteria after 3 h of incubation compared to those of the controls without linezolid (means ± standard deviations). *, P < 0.05 compared with control.
In summary, linezolid penetrates both phagocytes and nonphagocytic cells, reaching intracellular concentrations slightly greater than the extracellular ones. Linezolid showed slight intracellular activity against S. epidermidis at high intracellular concentrations.
Acknowledgments
This work was partially supported by a grant from Pharmacia.
REFERENCES
- 1.Brickner, S. J., D. K. Hutchinson, M. R. Barbachyn et al. 1996. Synthesis and antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential tratment of multidrug-resistant gram-positive bacterial infections. J. Med. Chem. 36:673-679. [DOI] [PubMed] [Google Scholar]
- 2.Clemett, D., and A. Marrkam. 2000. Linezolid. Drug 59:815-827. [DOI] [PubMed] [Google Scholar]
- 3.Diekema, D. I., and R. N. Jones. 2000. Oxazolidinones: a review. Drugs 59:7-16. [DOI] [PubMed] [Google Scholar]
- 4.Dresser, L. D., and M. J. Rybak. 1998. The pharmacologic and bacteriologic properties of oxazolidinones, a new class of synthetic antimicrobials. Pharmacotherapy 18:456-462. [PubMed] [Google Scholar]
- 5.Ford, C. W., J. C. Hamel, D. M. Wilson, J. K. Moerman, D. Stapert, R. J. Yancey, Jr., D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vivo activities of U-100592 and U-100766, novel oxazolidinone antimicrobial agents, against experimental bacterial infections. Antimicrob. Agents Chemother. 40:1508-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.French, G. 2001. Linezolid. Int. J. Clin. Pract. 55:59-63. [PubMed] [Google Scholar]
- 7.García, I., A. Pascual, M. C. Guzman, and E. J. Perea. 1994. Uptake and intracellular activity of sparfloxacin in human polymorphonuclear leukocytes and tissue culture cells. Antimicrob. Agents Chemother. 36:1053-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klempner, M. S., and B. Styrt. 1981. Clindamycin uptake by human neutrophils. J. Infect. Dis. 144:472-475. [DOI] [PubMed] [Google Scholar]
- 9.Pascual, A. 1995. Uptake and intracellular activity of antimicrobial agents in phagocytic cells. Rev. Med. Microbiol. 6:228-235. [Google Scholar]
- 10.Pascual, A., I. Garcia, and E. J. Perea. 1989. Fluorometric measurement of ofloxacin by human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 33:653-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pascual, A., D. Tsakayama, J. Kovarik, G. Gekker, and P. K. Peterson. 1987. Uptake and activity of rifapentine in human peritoneal macrophages and polymorphonuclear leukocytes. Eur. J. Clin. Microbiol. Infect. Dis. 6:152-157. [DOI] [PubMed] [Google Scholar]
- 12.Pascual, A., I. García, S. Ballesta, and E. J. Perea. 1997. Uptake and intracellular activity of trovafloxacin in human phagocytes and tissue-cultured epithelial cells. Antimicrob. Agents Chemother. 41:274-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinabarger, D. L., K. Marotti, R. W. Murray, A. H. Lin, E. P. Melchior, S. M. Swaney, D. S. Dunyak, W. F. Demyan, and J. M. Buysse. 1997. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob. Agents Chemother. 41:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swaney, S. M., H. Aoki, M. C. Ganoza, and D. L. Shinabarger. 1998. The oxazolidinone inhibits initiation of protein synthesis in bacteria. Antimicrob. Agents Chemother. 42:3251-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zurenko, G. E., B. H. Yagi, R. D. Schaadt, J. W. Allison, J. O. Kilburn, S. E. Glickman, D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]