Abstract
Site saturation mutagenesis of the 238 position in the SHV β-lactamase was performed to identify the complete sequence requirements needed for the extended spectrum β-lactamase (ESBL) phenotype. MICs (in micrograms per milliliter) in an isogenic background, Escherichia coli DH10B, demonstrated that the Gly238Ala mutation conferred the most resistance to penicillins and cephalosporins. The absolute increase in resistance was greatest against cefotaxime for the Gly238Ala mutant (0.06 to 8 μg/ml). Except for the strain possessing the Gly238Pro β-lactamase, ceftazidime MICs were also elevated. None of the mutant SHV β-lactamases were expressed in as great an amount as the wild-type β-lactamase. Kinetic analysis of the Gly238Ala mutant revealed that penicillin and cephalosporin substrates have a lower Km for the enzyme because of this mutation. Ampicillin and piperacillin MICs were inversely proportional to the side chain volume of the amino acid in cases larger than Ser, suggesting that steric considerations may be a primary requirement for penicillin resistance. Secondary structural effects explain increased resistance to oxyiminocephalosporins. Based upon this study, we anticipate that additional mutations of Gly238 in the SHV β-lactamase will continue to be discovered with an ESBL (ceftazidime or cefotaxime resistant) phenotype.
Among gram-negative bacteria, the production of β-lactamase enzymes still remains the most important mechanism of resistance to β-lactam antibiotics. The prevalent β-lactamase enzymes in Escherichia coli and Klebsiella pneumoniae are the TEM-1 and the SHV-1 β-lactamases (16). Gram-negative bacteria expressing the TEM-1 and SHV-1 β-lactamases are highly resistant to the aminopenicillins (ampicillin and amoxicillin), ureidopenicillins (piperacillin), and cephalothin. Alterations of single amino acids in TEM-1 and SHV-1 β-lactamase drastically modify the resistance phenotype of these β-lactamases. The point mutations that have received the most attention are the substitutions that result in increased resistance to ceftazidime, cefotaxime, and the monobactam, aztreonam (3, 21). These altered β-lactamases are referred to as extended-spectrum β-lactamases (ESBLs). Gram-negative bacteria harboring ESBLs have seriously threatened our ability to treat many infections in nursing home and hospital settings (2, 26, 27).
In nature, the substitutions of Ser, Ala, or Asp for Gly at the Ambler position ABL 238 are mutations in SHV β-lactamase that confer resistance to advanced generation cephalosporins (1, 3). There are currently 21 TEM and 12 SHV β-lactamase variants with the substitution Gly238Ser (http://www.lahey.org/studies/webt.htm). Numerous hypotheses have been advanced to explain why the Gly238Ser substitution results in significant resistance to broad-spectrum cephalosporins (6, 9, 12, 13, 22, 28). A molecular model proposed by Huletsky et al. maintains that Gly238Ser, due to steric conflict with residue Met69, expands the active site cavity by moving the b3 β-strand by 1 to 2 Å (9). This wider binding cavity is now able to form more favorable H-bonds with ceftazidime and improves the affinity for antibiotic binding. A second model, based upon molecular mechanics of TEM-19 (Gly238Ser), proposes that the Ser side chain points into the active site between N170 Oδ and the carbonyl oxygen of Ala237, a position that facilitates H-bond formation with the oxime group of ceftazidime (22). Saves et al. maintain that the change brought about by the hydroxyl group of Gly238Ser could reposition Asn170 in the Ω loop and displace this portion of the enzyme affecting both affinity for substrates and catalysis (28). Most recently, it has also been suggested that hydrogen bonding that results from the Gly-to-Ser substitution is important but is not required to achieve hydrolytic activity against broad-spectrum cephalosporins (6). Optimal side chain volume is necessary. This model states that an H-bond from the Ser hydroxyl of Gly238Ser stabilizes the position of the Ω loop for hydrolysis of broad-spectrum cephalosporins. The H-bond forms with Asn170, rather than the oxime group of the antibiotic.
The crystal structure of the ESBL-type TEM β-lactamase, TEM-52 (Glu104Lys-Met182Thr-Gly238Ser) confirms the notion that a major structural change has occurred as a result of the Gly238Ser substitution (17). The OH of Gly238Ser forms two new intramolecular H-bonds with residue 243. This moves the b3 β-stand by 2.8 Å.
Because of the significant role Ambler position 238 plays in both TEM and SHV ESBLs, we sought to understand how additional amino acid substitutions at this position in SHV could modify catalytic function of the enzyme and phenotype of the resistant bacteria. SHV was chosen for study because the Gly238Ser substitution confers a higher level of resistance to broad-spectrum cephalosporins than in TEM (29). To this end, we performed site saturation mutagenesis of Ambler position 238 and characterized the phenotypes of these variants. This approach allows an in-depth analysis of the sequence requirements for resistance and permits an appreciation of the spectrum of mutations that result in functional β-lactamases. In keeping with the notion that expression levels or quantity of β-lactamase produced is also important in understanding the development of resistance, we assayed SHV β-lactamase production. We purified and determined kinetic parameters for the Gly238Ala β-lactamase, since this mutant demonstrated the greatest resistance to penicillins and cephalosporins, and we compared this mutant to the Gly238Ser β-lactamase (7). This approach allowed us to appreciate the consequences of these mutations in SHV and anticipate the discovery of important amino acid variants at Ambler position 238.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The chromosomal SHV-1 β-lactamase with its native promoter and ribosomal binding site was cloned in the recombinant phagemid vector, pBCSK(−) (Stratagene, La Jolla, Calif.) as previously described (25). Plasmid DNA was obtained from E. coli cells by using Wizard purification kits (Promega, Madison, Wis.).
Mutagenesis.
PCR-based site saturation and site-directed mutageneses were performed by using Stratagene's QuikChange mutagenesis kit. We constructed two complementary degenerate oligonucleotides (Genosys Biotechnologies, The Woodlands, Tex.) at amino acid position 238 (Table 1) and performed mutagenesis (7). E. coli XL1-Blue cells were transformed with the mutagenic DNA to repair nicks. Colonies were plated on Luria-Bertani (LB) agar containing chloramphenicol (Sigma) at 20 μg/ml, and colonies were screened for the desired mutations. After verification, mutagenic DNA from one colony was transformed into E. coli DH10B. All experimental data and further sequence analysis was done in E. coli DH10B or on plasmid DNA from DH10B cells. After we sequenced 40 transformants, we determined that four variants were still needed (-Cys, -Pro, -Gln, and -Asp). Four sets of oligonucleotides were designed based on common codon usage, and the remaining mutants were constructed by using site-directed mutagenesis and the QuikChange Mutagenesis Kit as described above (Table 1) (7).
TABLE 1.
Oligonucleotides used for mutagenesis
| Oligonucleotidea | Sequence |
|---|---|
| G238NNS-1 | 5′-CGCCGATAAGACCGGAGCT(AGCT)(AGCT)(GC)GAGCGGGGTGCGCGCG-3′ |
| G238NNS-2 | 5′-CGCGCGCACCCCGCTC(GC)(AGCT)(AGCT)AGCTCCGGTCTTATCGGCG-3′ |
| G238Asp-1 | 5′-CGCCGATAAGACCGGAGCTGACGAGCGGGGTGCGCGCG-3′ |
| G238Asp-2 | 5′-CGCGCGCACCCCGCTCGTCAGCTCCGGTCTTATCGGCG-3′ |
| G238Cys-1 | 5′-CGCCGATAAGACCGGAGCTTGCGAGCGGGGTGCGCGCG-3′ |
| G238Cys-2 | 5′-CGCGCGCACCCCGCTCGCAAGCTCCGGTCTTATCGGCG-3′ |
| G238Gln-1 | 5′-CGCCGATAAGACCGGAGCTCAGGAGCGGGGTGCGCGCG-3′ |
| G238Gln-2 | 5′-CGCGCGCACCCCGCTCCTGAGCTCCGGTCTTATCGGCG-3′ |
| G238Pro-1 | 5′-CGCCGATAAGACCGGAGCTCCGGAGCGGGGTGCGCGCG-3′ |
| G238Pro-2 | 5′-CGCGCGCACCCCGCTCCGGAGCTCCGGTCTTATCGGCG-3′ |
N represents equal amounts of A, C, G, and T nucleotides. S represents equal amounts of G and C nucleotides.
DNA sequencing.
We performed DNA sequencing with an ALF Express automated DNA sequencer (Amersham Pharmacia Biotech, Piscataway, N.J.) by using the Thermo Sequenase fluorescent-labeled primer cycle sequencing kit (7).
MICs.
E. coli DH10B expressing the blaSHV genes were phenotypically characterized by LB agar dilution MICs by using a Steers replicator that delivered 104 CFU/spot. Antibiotics (ampicillin, piperacillin, cephaloridine, cephalothin, and cefotaxime) were obtained from Sigma Chemical Co. (St. Louis, Mo.). Ceftazidime was obtained from a commercial supplier (Glaxo-Wellcome Research). Aztreonam and cefepime were obtained from Bristol-Meyers Squibb Company (Princeton, N.J.). Sodium tazobactam was a generous gift from Wyeth-Ayerst (Pearl River, N.Y.). Concentrations used for determining MICs were in micrograms per milliliter. MICs were performed a minimum of three times for each antibiotic.
Determination of steady-state expression by using slot blots and an ELISA.
We used two procedures to assess and confirm steady-state expression levels using our polyclonal anti-SHV antibody: slot blotting and an enzyme-linked immunosorbent assay (ELISA) (8). In brief, a 3-ml culture was grown to an optical density at 600 nm (OD600) of 0.8. After a boiling step, a 10-μl aliquot was loaded onto a polyvinylidene difluoride membrane by using the Bio-Rad Slot Blot apparatus (Hercules, Calif.). Each of the strains possessing the 19 variants and the wild-type β-lactamase were assayed for in vivo steady-state expression levels by probing them with 1 μg/ml of purified anti-SHV antibody and horseradish peroxidase-conjugated protein G (Bio-Rad) as previously reported (7). Chemiluminescent detection was performed by using an ECL Kit (Amersham Pharmacia Biotech). Western blotting was performed according to a previously described protocol (7).
In order to quantify the amount of SHV-1 and mutant β-lactamases that are produced at steady state, our polyclonal antibody was used in an ELISA format that we developed in our laboratory (8). Ninety-six-well Immulon-4 enzyme immunoassay plates were coated overnight with 4 μg of anti-SHV-1 polyclonal antibody/ml diluted in carbonate buffer (pH 9.5). Plates were washed six times with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (Bio-Rad) and blocked with 5% bovine serum albumin in PBS for 2 h at room temperature. Purified SHV-1 β-lactamase was serially diluted at fixed concentrations to be used as standards for this ELISA. Then, 100-μl aliquots of overnight bacterial cultures containing mutant enzymes were boiled for 10 min and serially diluted. These dilutions were applied for 2 h to the enzyme immunoassay plates, washed again, and incubated for an additional 1 h with 2 μg of biotinylated anti-SHV-1 polyclonal antibody/ml. Concentrations of coating and biotinylated detecting antibody were varied initially to empirically determine the best concentrations for determining the presence of SHV β-lactamase (8). Between all steps after sample incubation, six washes were carried out with 0.05% Tween 20-PBS. Plates were then incubated with a 1:3,000 dilution of streptavidin-horseradish peroxidase (Zymed Laboratories, South San Francisco, Calif.) for 30 min, followed by development with o-phenylenediamine and H2O2 diluted in citric acid buffer (pH 5.5; Sigma). Development was terminated by the addition of H2SO4 to a concentration of 0.5 M. The OD492 values were determined and compared to serially diluted, purified SHV-1 β-lactamase used as an internal point of reference for concentration calculation.
Radiolabeling, immunoprecipitation, and autoradiography of SHV β-lactamases.
Pulse-chase experiments were performed as follows. Bacterial cultures were grown to an OD600 of 0.5 under normal conditions in LB broth. Cells were then labeled with [35S]methionine at a concentration of 30 μCi/ml in a 2-ml volume. After 10 min of pulse-labeling, excess unlabeled l-methionine (2.5 mg/ml) was added. At 30 and 300 s after the chase was begun, 1 ml of culture was removed. We lysed the cells by boiling them for 5 min. After the boiling step, 1 mM phenylmethylsulfonyl fluoride (Sigma) was added, and the lysates were cleared by centrifugation at 4°C for 10 min. The supernatant was removed, and the β-lactamase was immunoprecipitated by addition of 10 μg of anti-SHV antibody/ml with incubation for 1 h at 4°C on a rotating disk. After the addition of 20 μl of protein G-agarose beads (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), samples were incubated for an additional hour with constant rotation at 4°C. The beads were washed four times with 0.5 ml of radioimmunoprecipitation assay buffer (50 mM Tris HCl, 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 1 mM dithiothreitol; pH 7.5). The agarose bead pellets were suspended in 10 μl of 2× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer and boiled. Samples were then resolved on a 5% stacking-12% separating SDS-PAGE gel, dried, and autoradiographed to visualize radiolabeled proteins.
β-Lactamase purification.
SHV-1, Gly238Ser, and Gly238Ala β-lactamases were purified from E. coli DH10B according to a previously published method employing preparative isoelectric focusing (15). We assessed the purity of each enzyme by SDS-PAGE as described above. Gels were stained with Coomassie brilliant blue R250 (Fisher, Pittsburgh, Pa.) to visualize proteins. Protein concentrations were determined with Bio-Rad's Protein Assay with bovine serum albumin as a standard.
Kinetics.
Michaelis constants of the Gly238Ala β-lactamase were determined by continuous assays at room temperature by using a Agilent 8452 diode array spectrophotometer. Kinetic parameters were previously determined for SHV-1 and Gly238Ser β-lactamases (7). Measurements were obtained with ampicillin (Δɛ235 = −900 M−1 cm−1), benzylpenicillin (Δɛ540 = −1,140 M−1 cm−1), cephaloridine (Δɛ260 = −10,200 M−1 cm−1), nitrocefin (Δɛ472 = 17,400 M−1 cm−1) (Becton Dickinson, Cockeysville, Md.), cefotaxime (Δɛ264 = −7,250 M−1 cm−1), and ceftazidime (Δɛ260 = −8,660 M−1 cm−1). The kinetic parameters, Vmax and Km, were determined by a nonlinear least-squares fit of the data to the Michaelis-Menten equation calculated by using the program Enzfitter (Sigma). The relative hydrolytic activity (in micromolar concentration/second/milligram of protein) was determined by measuring the hydrolysis of nitrocefin by using crude enzyme preparations of β-lactamases obtained according to a previously described method (19).
Molecular representations.
Kuzin et al. determined the atomic structure of the SHV-1 β-lactamase (Fig. 1) (11). Coordinates are available from the Protein Data Bank (entry 1 SHV) at Rutgers University (www.rcsb.org/pdb/). We constructed a representation of the crystallographic structure of the SHV-1 β-lactamase in the environment around Gly238 with the program WebLab ViewerLite 3.5 (www.accelrys.com/products/).
FIG. 1.
Molecular representation of the SHV-1 β-lactamase-binding cavity generated by using coordinates for SHV-1, deposited in the Protein Data Bank, and WebLab ViewerLite 3.5. We performed site saturation mutagenesis of Gly238. Amino acids Ser70, Asn170, Ala237, and Glu240 are also shown.
RESULTS
Mutagenesis.
The site of mutagenesis and the flanking region upstream and downstream of the mutation site in each variant was verified by DNA sequencing and confirmed to contain only the specified mutation(s). The degenerate oligonucleotides used for the mutagenesis reaction yielded 15 unique mutants from 40 screened transformants. The remaining four mutants at ABL position 238 (Gly238Cys, -Pro, -Gln, and -Asp) were constructed by using site-directed mutagenesis. For each amino acid substitution the most common codon, as determined by analysis of blaSHV-1 AF124984 (our entry into GenBank from which the structure of SHV-1 was determined) was used. The exceptions to this were for amino acids—Thr (ACG), Asn (AAC), Glu (GAG), Lys (AAG), Phe (TTC), and Tyr (TAC)—which were the second most common codons used for these amino acids in blaSHV-1.
Antibiotic susceptibility testing.
All Gly238 mutant β-lactamases expressed in E. coli DH10B were phenotypically characterized (Table 2). We listed each mutant by increasing amino acid side chain volume. Compared to the wild-type SHV-1 β-lactamase expressed in E. coli, the MICs against ampicillin were highest for the Ala and Ser mutants (8,000 to 16,000 μg/ml). Slightly lower levels of ampicillin resistance were demonstrated for the Thr, Asn, Val, Ile, Met, His, Lys, and Cys variants (1,000 to 8,200 μg/ml). Eight mutant β-lactamases possessed lower levels of resistance (128 to 512 μg/ml): Gly238Tyr, -Arg, -Phe, -Trp, -Leu, -Glu, -Asp, and -Gln. The strain most susceptible to ampicillin was the strain possessing the Gly238Pro mutant β-lactamase (32 μg/ml). For the most part, piperacillin resistance closely paralleled ampicillin resistance. Again, the Ala and Ser mutants were highly resistant and the Gly238Pro substitution in E. coli was the most susceptible.
TABLE 2.
MICs
| aaa | aa side chain vol (Å3)b | MIC (μg/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | Piperacillin | Cephaloridine | Cephalothin | Cefotaxime | Ceftazidime | Aztreonam | Cefepime | ||
| G | 16,000 | 2,000 | 128 | 32 | 0.06 | 1 | 1 | 0.5 | |
| A | 28.5 | 8,200 | 2,000 | 256 | 512 | 8 | 8 | 4 | 2 |
| S | 28.9 | 8,200 | 1,000 | 16 | 64 | 8 | 8 | 1 | 0.5 |
| C | 48.4 | 4,100 | 512 | 16 | 64 | 2 | 8 | 4 | 2 |
| P | 52.6 | 32 | 8 | 4 | 4 | 0.06 | 0.03 | 0.5 | 0.06 |
| D | 51.0 | 128 | 64 | 4 | 4 | 0.125 | 4 | 0.5 | 0.125 |
| T | 56.0 | 8,200 | 1,000 | 16 | 16 | 4 | 8 | 0.5 | 0.25 |
| N | 54.0 | 4,100 | 512 | 32 | 128 | 4 | 8 | 2 | 1 |
| V | 79.9 | 2,000 | 256 | 4 | 4 | 0.5 | 4 | 0.5 | 0.06 |
| E | 78.3 | 512 | 128 | 4 | 4 | 0.5 | 4 | 0.5 | 0.06 |
| Q | 83.7 | 512 | 128 | 4 | 4 | 2 | 8 | 0.5 | 0.25 |
| H | 93.1 | 1,000 | 256 | 16 | 32 | 0.5 | 4 | 1 | 1 |
| I | 106.6 | 2,000 | 512 | 8 | 4 | 2 | 4 | 0.5 | 0.25 |
| M | 102.8 | 2,000 | 256 | 8 | 16 | 2 | 8 | 0.5 | 0.125 |
| L | 106.6 | 512 | 256 | 16 | 32 | 0.5 | 8 | 0.5 | 1 |
| K | 108.5 | 2,000 | 256 | 8 | 16 | 1 | 8 | 0.5 | 0.125 |
| F | 129.8 | 512 | 256 | 32 | 32 | 0.25 | 4 | 0.5 | 1 |
| Y | 133.5 | 256 | 128 | 8 | 8 | 0.125 | 8 | 0.125 | 0.06 |
| R | 113.3 | 512 | 128 | 16 | 64 | 1 | 8 | 2 | 0.5 |
| W | 167.7 | 128 | 128 | 4 | 4 | 0.25 | 8 | 0.125 | 0.25 |
aa, amino acid.
Gly is taken as the reference amino acid. All volumes listed are relative to the side chain of Gly.
There were slight differences in MICs between the narrow-spectrum cephalosporins tested. For 8 of the 19 variants, cephalothin MICs were equal to or higher than those for SHV-1. The Gly238Ala variant was four dilutions more resistant than the wild-type SHV-1 β-lactamase-containing strain. The Gly238Ala variant also expressed the highest cephaloridine MICs, but the relative increase was less. In contrast to cephalothin MICs, 18 of 19 mutants were more susceptible to cephaloridine than the wild type.
In a similar pattern to the narrow-spectrum cephalosporins, susceptibility testing against cefotaxime and ceftazidime demonstrated two different patterns. The Ser- and Ala-containing variants were the most cefotaxime resistant (MICs = 8 μg/ml). A range of cefotaxime MICs were observed for the other variants. The observed pattern with ceftazidime was distinctly different. All variants (except for Gly238Pro) demonstrated similar levels of resistance (ceftazidime MICs ranged from 4 to 8 μg/ml). The Gly238Ala and -Cys mutants expressed in E. coli demonstrated greater aztreonam and cefepime resistance compared to Gly238Ser or the wild-type SHV-1 strain. None of the variants at the 238 position exhibited resistance to ampicillin- tazobactam (data not shown).
Determination of expression levels of SHV mutants expressed in E. coli DH10B by slot blot and ELISA.
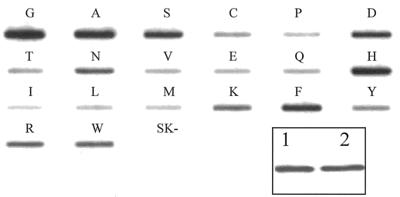
In order to show that mutations at Ambler position 238 did not significantly alter the ability of our polyclonal antibody to accurately assess levels of expression, we verified that purified wild-type enzyme and an identical amount of purified Gly238Ala β-lactamase were recognized equally by our polyclonal antibody in Western blot analysis (Fig. 2, inset). Polyclonal antibodies recognize multiple epitopes and are less likely to be influenced by a single point mutation.
FIG. 2.
Western blot and slot blot of SHV-1 β-lactamase and mutants at the 238 position. The Western blot and slot blot were probed with 1 μg of anti-SHV-1 antibody/ml and horseradish peroxidase-conjugated protein G (7). Single-letter designations for the Gly238 mutant β-lactamases are listed above each slot. SK− represents the strain E. coli DH10B with the vector pBCSK(−) without the SHV β-lactamase. (Inset) Western blot of SHV-1 and Gly238Ala variant of the SHV β-lactamase. Equal amounts of purified β-lactamase were loaded in each lane. Lane 1, SHV-1; lane 2, Gly238Ala. As evaluated by densitometry, SHV-1 and Gly238Ala are nearly equivalent.
Steady-state β-lactamase expression levels were qualitatively examined by slot blot analysis (Fig. 2). The wild type and the Ala, Ser, Asp, Asn, His, and Phe mutants expressed the largest amount of SHV β-lactamase. In contrast, the Pro, Val, Glu, Leu, Ile, and Met mutants were expressed at lower amounts than the wild-type β-lactamase. β-Lactamase expression did not directly correlate with levels of ampicillin or cefotaxime resistance.
To confirm our qualitative assessment of β-lactamase expression, we determined the amounts of SHV β-lactamase produced in each of the strains by a quantitative ELISA (Table 3). These data are in agreement with the pattern observed by using the slot blot format.
TABLE 3.
ELISA determination of β-lactamase production of Gly238 mutants
| aa variationa | SHV production (ng/ml)b |
|---|---|
| Gly (wt) | 370 |
| Ala | 151 |
| Ser | 90 |
| Cys | 7 |
| Pro | 6 |
| Asp | 101 |
| Thr | 13 |
| Asn | 45 |
| Val | 9 |
| Glu | 12 |
| Gln | 15 |
| His | 91 |
| Ile | 10 |
| Leu | 6 |
| Met | 9 |
| Lys | 14 |
| Phe | 56 |
| Tyr | 12 |
| Arg | 14 |
| Trp | 9 |
| SK− | 0.03 |
aa, amino acid; wt, wild type.
±6% error (8).
Pulse-chase analysis of SHV-1 and mutant β-lactamases.
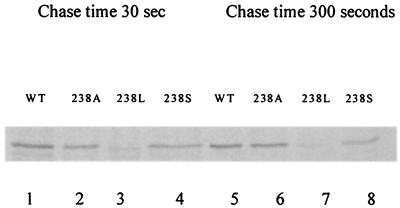
The variability in expression of the 238 mutants, as demonstrated by slot blot and ELISA, could result from decreased β-lactamase translation or increased turnover (18). To explore these possibilities, we chose to study the wild type and the Gly238Ser, Gly238Ala, and Gly238Leu mutants because of differing MICs and steady-state expression levels.
E. coli DH10B cells possessing the mutant β-lactamases were pulse-labeled with [35S]methionine at the same OD and chased in the presence of excess cold methionine for 30 and 300 s. The comparison of the wild-type enzyme, SHV-1 β-lactamase, with Gly238Ser, Gly238Ala, and Gly238Leu (Fig. 3) reveal that these variants of SHV β-lactamase are translated at different rates. The Gly238Leu mutant produced less β-lactamase under these conditions than did the wild type, the G238Ser, and the Gly238Ala β-lactamases. This pattern also paralleled steady-state expression levels as determined by the SHV slot blot and quantitative ELISA.
FIG. 3.
Pulse-chase analysis of SHV-1 and Gly238Ala, -Leu, and -Ser mutant β-lactamases. Pulse-chase was terminated at 30 and 300 s. The β-lactamase was immunoprecipitated from solubilized E. coli DH10B cells and resolved by SDS-PAGE and autoradiographed. Lanes 1 to 4, 30-s cold methionine chase; lanes 5 to 8, 300-s cold methionine chase.
Kinetics.
We purified Gly238Ala β-lactamase to >90% homogeneity, as verified by SDS-PAGE, and compared the Michaelis constants to purified wild-type and Gly238Ser β-lactamases. The kinetic parameters are summarized in Table 4. Compared to SHV-1 β-lactamase, the Gly238Ser and Gly238Ala enzymes demonstrated lower Km values for penicillins (benzylpenicillin and ampicillin) and for cephaloridine. There was also a modest decrease in turnover numbers (kcat) and catalytic efficiencies (kcat/Km) for benzylpenicillin, ampicillin, cephaloridine (-Ala only), and nitrocefin.
TABLE 4.
Michaelis constants
| Antibiotic and enzyme | Km (μM) | kcat (s−1) | kcat/Km (10−6 M−1 s−1) |
|---|---|---|---|
| Benzylpenicillin | |||
| SHV-1 | 28 ± 6 | 980 ± 45 | 35 ± 6 |
| Gly238Ser | 13 ± 5 | 32 ± 2 | 2.5 ± 0.8 |
| Gly238Ala | 3.40 ± 0.03 | 37.0 ± 0.1 | 11 ± 0.1 |
| Ampicillin | |||
| SHV-1 | 120 ± 27 | 2,400 ± 214 | 20 ± 2.7 |
| Gly238Ser | 10 ± 2 | 30 ± 2 | 3.0 ± 0.4 |
| Gly238Ala | 31.0 ± 4.4 | 180 ± 14 | 5.8 ± 0.37 |
| Cephaloridine | |||
| SHV-1 | 50 ± 10 | 150 ± 13 | 3 ± 0.34 |
| Gly238Ser | 40 ± 13 | 500 ± 144 | 10 ± 0.2 |
| Gly238Ala | 16 ± 3 | 36.0 ± 0.2 | 2.3 ± 0.4 |
| Nitrocefin | |||
| SHV-1 | 5.0 ± 0.9 | 142 ± 4 | 28.4 ± 4 |
| Gly238Ser | 10 ± 2 | 37 ± 2 | 4 ± 0.5 |
| Gly238Ala | 3.2 ± 0.3 | 46 ± 1 | 14 ± 0.3 |
| Cefotaxime | |||
| SHV-1 | NMa | NM | |
| Gly238Ser | 9 ± 2 | 6.0 ± 0.4 | 0.6 ± 0.1 |
| Gly238Ala | 12.4 ± 0.8 | 4.40 ± 0.01 | 0.4 ± 0.02 |
NM, not measurable.
We assayed relative hydrolytic activities based upon the hydrolysis of 100 μM nitrocefin (Table 5). We observed a significant reduction in the ability of each mutant enzyme to hydrolyze nitrocefin compared to the wild type.
TABLE 5.
Relative hydrolytic activity (with 100 μM nitrocefin) of Gly238 β-lactamase mutants
| aa variationa | RHA (μM/s/mg of protein)b |
|---|---|
| Gly (wt) | 281.3 |
| Ala | 20.8 |
| Ser | 4.34 |
| Cys | 0 |
| Pro | 0.64 |
| Asp | 4.2 |
| Thr | 13.8 |
| Asn | 28.9 |
| Val | 9.4 |
| Glu | 37.5 |
| Gln | 37.8 |
| His | 12.6 |
| Ile | 13.7 |
| Leu | 0.9 |
| Met | 38.5 |
| Lys | 16.0 |
| Phe | 7.2 |
| Tyr | 2.3 |
| Arg | 8.8 |
| Trp | 1.5 |
aa, amino acid; wt, wild type.
RHA, relative hydrolytic activity (±10% error). The mean of three independent determinations is given.
DISCUSSION
Our experiments are the first systematic analysis of the effect of all amino acid substitutions in SHV-1 β-lactamase at Ambler position 238 in an isogenic E. coli background. In a previous study of SHV-1 mutagenesis, we observed that the Gly238Ser mutation preserved broad-spectrum penicillin, cephalosporin, and oxyimino-cephalosporin resistance (7). Western blots also indicated that variants with the Gly238Ser mutation (Gly238Ser-Glu240Lys and Asp104Lys-Gly238Ser-Glu240Lys) produced more β-lactamase than other SHV variants. The goal of the present study was to extend that analysis: to use site saturation mutagenesis to predict which mutations would result in an ESBL phenotype at the 238 position and what would be the consequence of these mutations on phenotype, catalysis, and β-lactamase expression.
From the MIC determinations, it is clear that nearly all amino acid substitutions in SHV at position 238 preserve clinically important ampicillin and piperacillin resistance (MICs ≥ 128 μg/ml in 18 of 20 variants). Previous work performed by Lenfant et al. found that TEM with multiple substitutions at Gly238 (the -Ala, -Ser, and -Thr substitutions) were amoxacillin and cefotaxime resistant; the -Cys, -Lys, -His, -Phe, and -Pro β-lactamases were inactive (14). Why differences between SHV and TEM β-lactamase variants at the 238 position exist is not clear.
Our results show that ampicillin resistance decreases for nearly all amino acid substitutions. Cephalosporin resistance follows a slightly different pattern. Maximal cephalosporin (cephaloridine, cephalothin, ceftazidime, cefotaxime, and cefepime) and monobactam resistance is achieved with the Gly238Ala mutation expressed in E. coli DH10B. These MIC data are consistent with the studies performed with SHV-13 (Leu35Glu and Gly238Ala) and SHV-18 (Ile8Phe-Arg43Ser-Gly238Ala-Glu240Lys) (24, 30). The difference we observed in MICs between cephalosporins and penicillins raises the question: is the ABL238 position “cephalosporin specific” in SHV (i.e., greater cephalosporinase activity versus penicillinase activity)? In TEM, Ala237Thr and Glu240Cys assume this role (4, 5).
To better understand whether amino acid substitutions influence the levels of β-lactamase expression and β-lactam resistance in the 238 variants, we performed immunoblot analysis and assayed SHV β-lactamase quantities by using a specific ELISA. Both techniques reveal that in vivo amounts of SHV β-lactamase expression vary in these mutants. The pattern is not directly correlated with levels of resistance against penicillins, narrow-spectrum cephalosporins, or the oxyiminocephalosporins. We attempted to determine whether these differences in expression were due to differences in β-lactamase translation or protein stability (as determined by steady-state expression). To our surprise, the pulse-chase experiment suggests that, at least for the variant β-lactamases chosen for study, there seems to be a qualitative difference in translational efficiency. In contrast, studies performed in TEM examining strains possessing β-lactamases with Ω loop mutations did not demonstrate a difference in translational efficiency. All TEM variants were produced at the same rate (18, 20). It was proposed that differences in TEM steady-state expression are due to the instability of the mutant β-lactamases. Studies performed by Raquet et al., testing the stability of TEM ESBLs, confirmed that Gly238Ser is a less stable enzyme, as assessed by thermal stability, trypsin proteolysis, and equilibrium denaturation by guanidine hydrochloride (23). Our slot blots, quantitative ELISA, and pulse-chase data show that there is variability in expression and translational efficiency in SHV. Factors responsible for the observation of decreased expression of Gly238 mutants in SHV mandate further investigation.
Using the Gly238Ser and -Ala mutants of SHV as model enzymes for kinetic analysis (most robust MICs), we see that the Km for benzylpenicillin, ampicillin, and cephaloridine are much lower than the wild-type enzyme. This suggests that there is a change in the geometry of the active site that improves binding. We do not suspect that this change influences the rate of deacylation (22). There is also a uniform reduction in kcat and kcat/Km (cephaloridine, Ala only) values. A similar pattern was observed with the Gly238Ser and Gly238Ala mutants of TEM for penicillin substrates (6).
Comparing the relative hydrolytic activity of each of the SHV 238 β-lactamases, we see that each mutation results in significant reduction in activity. This may reflect a combined effect of decreased relative β-lactamase steady-state expression and/or catalytic efficiency (Vmax/Km). None of the mutants are as active as the wild-type β-lactamase.
The crystal structure of SHV-1 and TEM-52 (Glu104Lys-Met182Thr-Gly238Ser) served as guides to understanding our results. The 238 residue sits on the b3 β-strand in SHV-1 and TEM and is in close proximity to Asn170, Met69, and Ala 237 (Fig. 1). In TEM-52, the first TEM ESBL to be crystallized, the hydroxyl of the Gly238Ser substitution forms two new H bonds: one to the Ser243 backbone amide and one to the Ser243 hydroxyl group (17). This widens the active site and may facilitate binding of “bulky” oxyiminocephalosporin substrates. With this structural finding in mind, our data reveal two important trends. First, as the side chain volume of the amino acid at position 238 increases beyond the size of Ala and Ser, there is a striking decrease in ampicillin and piperacillin resistance (Table 2). The volume of the side chain residue in SHV-1 plays a role in determining ampicillin and piperacillin resistance levels as it does in TEM (6). When the cephalosporin antibiotics are examined in this manner, the highest overall MICs are seen with -Ala and -Asn. For cephems, we propose that both the volume of the amino acid side chain and the intramolecular interactions of the residue at 238 determine resistance. It is conceivable that amino acid substitutions of the correct size that permit movement of the b3 β-strand to a more favorable position to accommodate the entry of a substrate with a bulky R group (broad-spectrum cephalosporins), results in cephalosporin resistance. Because of this flexibility, we propose that the position of the oxyanion hole (as defined by the amide groups of Ala237 and Ser70) is preserved and catalysis occurs (10). The aromatic branched amino acids (-Tyr, -Trp, and -Phe) and -Pro confer the least flexibility to the b3 β-sheet. These substitutions translate to the greatest susceptibility to ampicillin and cephalosporin antibiotics. It is very noteworthy that resistance to ceftazidime does not depend exclusively on volume or nature of the amino acid side chain. In each case, we see a β-lactamase conferring resistance to ceftazidime independent of amino acid volume, H-bonding capacity or polarity (MICs = 8 μg/ml). The structural and spatial movement from each mutation still permits binding and hydrolysis of ceftazidime.
After we tested the complete amino acid array of substitutions, we conclude (i) that multiple substitutions can result in ESBL-type SHV β-lactamases and (ii) that a number of important SHV β-lactamase properties are altered. Seven variants of SHV at 238 (-Ser, -Ala, -Thr, -Asn, -Met, -Cys, and -Ile) increase cefotaxime resistance five dilutions (2-log increase) and still have ampicillin MICs >1,024 μg/ml. Nearly all mutations resulted in ceftazidime resistance. Our data anticipate that ESBL variants of SHV will be found that possesses many different substitutions at this site. Most likely, the mutations that require only a single nucleotide change, preserve common codon usage, and are expressed well will be the first to arise (e.g., Gly238Ser, -Ala, and -Asp). Gly238Asn, although highly resistant, would require two changes. A formal explanation for why the ESBL phenotype at 238 occurs must take into account the nature of the active site cavity, the substrate being hydrolyzed, the optimal amino acid side chain volume, and b3 β-strand movement or flexibility. Our experiments anticipate which mutants are likely to arise and provide functional support to the notion that steric movement of the b3 β strand plays a critical role in the alteration of substrate specificity for penicillins and cephalosporins in SHV β-lactamase. Why SHV is different than TEM, how SHV β-lactamase expression is altered, and how this may influence the clinical discovery of these novel variants remain challenging questions.
Acknowledgments
This work was supported by grants from the National Institutes on Aging to R.A.B. (5 K08 AG 00684-05), from the Veterans Affairs Medical Center Merit Review Program, and from the Geriatric Research Education and Clinical Center. K. Hujer was supported by a grant from Merck Research Laboratories.
We appreciate the comments of Louis B. Rice, Piet DeBoer, and M. Macguire.
REFERENCES
- 1.Ambler, R. P., A. F. Coulson, J. M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276(Pt. 1):269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford, P. A., C. Urban, A. Jaiswal, N. Mariano, B. A. Rasmussen, S. J. Projan, J. J. Rahal, and K. Bush. 1995. SHV-7, a novel cefotaxime-hydrolyzing β-lactamase, identified in Escherichia coli isolates from hospitalized nursing home patients. Antimicrob. Agents Chemother. 39:899-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantu III, C., W. Huang, and T. Palzkill. 1997. Cephalosporin substrate specificity determinants of TEM-1 β-lactamase. J. Biol. Chem. 272:29144-29150. [DOI] [PubMed] [Google Scholar]
- 5.Cantu III, C., W. Huang, and T. Palzkill. 1996. Selection and characterization of amino acid substitutions at residues 237 to 240 of TEM-1 β-lactamase with altered substrate specificity for aztreonam and ceftazidime. J. Biol. Chem. 271:22538-22545. [DOI] [PubMed] [Google Scholar]
- 6.Cantu III, C., and T. Palzkill. 1998. The role of residue 238 of TEM-1 β-lactamase in the hydrolysis of extended-spectrum antibiotics. J. Biol. Chem. 273:26603-26609. [DOI] [PubMed] [Google Scholar]
- 7.Hujer, A. M., K. M. Hujer, and R. A. Bonomo. 2001. Mutagenesis of amino acid residues in the SHV-1 β-lactamase: the premier role of Gly238Ser in penicillin and cephalosporin resistance. Biochim. Biophys. Acta 1547:37-50. [DOI] [PubMed] [Google Scholar]
- 8.Hujer, A. M., M. G. P. Page, M. S. Helfand, B. Yeiser, and R. A. Bonomo. 2002. Development of a sensitive and specific enzyme-linked immunosorbent assay for detecting and quantifying CMY-2 and SHV β-lactamases. J. Clin. Microbiol. 40:1947-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huletsky, A., J. R. Knox, and R. C. Levesque. 1993. Role of Ser-238 and Lys-240 in the hydrolysis of third-generation cephalosporins by SHV-type β-Lactamases probed by site-directed mutagenesis and three-dimensional modeling. J. Biol. Chem. 268:3690-3697. [PubMed] [Google Scholar]
- 10.Knox, J. R. 1995. Extended-spectrum and inhibitor-resistant TEM-type β-lactamases: mutations, specificity, and three-dimensional structure. Antimicrob. Agents Chemother. 39:2593-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuzin, A. P., M. Nukaga, Y. Nukaga, A. M. Hujer, R. A. Bonomo, and J. R. Knox. 1999. Structure of the SHV-1 β-lactamase. Biochemistry 38:5720-5727. [DOI] [PubMed] [Google Scholar]
- 12.Labia, R., A. Morand, K. Tiwari, J. Sirot, D. Sirot, and A. Petit. 1988. Interactions of new plasmid-mediated β-lactamases with third-generation cephalosporins. Rev. Infect. Dis. 10:885-891. [DOI] [PubMed] [Google Scholar]
- 13.Lee, K.-Y., J. D. Hopkins, T. F. O'Brien, and M. Syvanen. 1991. Gly238Ser substitution changes the substrate specificity of the SHV class A β-lactamase. Proteins Struct. Funct. Genet. 11:45-51. [DOI] [PubMed] [Google Scholar]
- 14.Lenfant, F., R. Labia, and J. M. Masson. 1990. Probing the active site of β-lactamase R-TEM1 by informational suppression. Biochimie 72:495-503. [DOI] [PubMed] [Google Scholar]
- 15.Lin, S., M. Thomas, D. M. Shlaes, S. D. Rudin, J. R. Knox, V. E. Anderson, and R. A. Bonomo. 1998. Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 β-lactamase, an SHV-family class A enzyme. Biochem. J. 333(Pt. 2):395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medeiros, A. A. 1997. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin. Infect. Dis. 24(Suppl. 1):S19-S45. [DOI] [PubMed] [Google Scholar]
- 17.Orencia, M. C., J. S. Yoon, J. E. Ness, W. P. Stemmer, and R. C. Stevens. 2001. Predicting the emergence of antibiotic resistance by directed evolution and structural analysis. Nat. Struct. Biol. 8:238-242. [DOI] [PubMed] [Google Scholar]
- 18.Palzkill, T., Q. Q. Le, K. V. Venkatachalam, M. LaRocco, and H. Ocera. 1994. Evolution of antibiotic resistance:several different amino acid substitutions in an active site loop alter the substrate profile of β-lactamase. Mol. Microbiol. 12:217-229. [DOI] [PubMed] [Google Scholar]
- 19.Paterson, D. L., L. B. Rice, and R. A. Bonomo. 2001. Rapid method of extraction and analysis of extended-spectrum β-lactamases from clinical strains of Klebsiella pneumoniae. Clin. Microbiol. Infect. 7:709-711. [PubMed] [Google Scholar]
- 20.Petrosino, J. F., and T. Palzkill. 1996. Systematic mutagenesis of the active site omega loop of TEM-1 β-lactamase. J. Bacteriol. 178:1821-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philippon, A., R. Labia, and G. A. Jacoby. 1989. Extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 33:1131-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raquet, X., J. Lamotte-Brasseur, E. Fonze, S. Goussard, P. Courvalin, and J. M. Frere. 1994. TEM β-lactamase mutants hydrolysing third-generation cephalosporins: a kinetic and molecular modeling analysis. J. Mol. Biol. 244:625-639. [DOI] [PubMed] [Google Scholar]
- 23.Raquet, X., M. Vanhove, J. Lamotte-Brasseur, S. Goussard, P. Courvalin, and J. M. Frere. 1995. Stability of TEM β-lactamase mutants hydrolyzing third generation cephalosporins. Proteins Struct. Funct. Genet. 23:63-72. [DOI] [PubMed] [Google Scholar]
- 24.Rasheed, J. K., G. J. Anderson, H. Yigit, A. M. Queenan, A. Domenech-Sanchez, J. M. Swenson, J. W. Biddle, M. J. Ferraro, G. A. Jacoby, and F. C. Tenover. 2000. Characterization of the extended-spectrum β-lactamase reference strain, Klebsiella pneumoniae K6 (ATCC 700603), which produces the novel enzyme SHV-18. Antimicrob. Agents Chemother. 44:2382-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice, L. B., L. L. Carias, A. M. Hujer, M. Bonafede, R. Hutton, C. Hoyen, and R. A. Bonomo. 2000. High-level expression of chromosomally encoded SHV-1 β-lactamase and an outer membrane protein change confer resistance to ceftazidime and piperacillin-tazobactam in a clinical isolate of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44:362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice, L. B., E. C. Eckstein, J. DeVente, and D. M. Shlaes. 1996. Ceftazidime-resistant Klebsiella pneumoniae isolates recovered at the Cleveland Department of Veterans Affairs Medical Center. Clin. Infect. Dis. 23:118-124. [DOI] [PubMed] [Google Scholar]
- 27.Rice, L. B., S. H. Willey, G. A. Papanicolaou, A. A. Medeiros, E. M. Eliopoulos, R. C. Moellering, Jr., and G. A. Jacoby. 1990. Outbreak of ceftazidime resistance caused by extended-spectrum β-lactamases at a Massachusetts chronic care facility. Antimicrob. Agents Chemother. 34:2193-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saves, I., O. Burlet-Schlitz, L. Maveyraud, J. P. Samama, J. C. Prome, and J. M. Masson. 1995. Mass spectral kinetic study of acylation and deacylation during the hydrolysis of penicillins and cefotaxime by β-lactamase TEM-1 and the G238S mutant. Biochemistry 34:11660-11667. [DOI] [PubMed] [Google Scholar]
- 29.Venkatachalam, K. V., W. Huang, M. LaRocco, and T. Palzkill. 1994. Characterization of TEM-1 β-lactamase mutants from positions 238 to 241 with increased catalytic efficiency for ceftazidime. J. Biol. Chem. 269:23444-23450. [PubMed] [Google Scholar]
- 30.Yuan, M., L. M. Hall, P. H. Savelkoul, C. M. Vandenbroucke-Grauls, and D. M. Livermore. 2000. SHV-13, a novel extended-spectrum β-lactamase, in Klebsiella pneumoniae isolates from patients in an intensive care unit in Amsterdam. Antimicrob. Agents Chemother. 44:1081-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]