Abstract
We have evaluated the anti-human immunodeficiency virus (HIV) activity of a series of natural and synthetic porphyrins to identify compounds that could potentially be used as microbicides to provide a defense against infection by sexually transmitted virus. For assays we used an epithelial HeLa-CD4 cell line with an integrated long terminal repeat-β-galactosidase gene. For structure-activity analysis, we divided the porphyrins tested into three classes: (i) natural porphyrins, (ii) metallo-tetraphenylporphyrin tetrasulfonate (metallo-TPPS4) derivatives, and (iii) sulfonated tetra-arylporphyrin derivatives. None of the natural porphyrins studied reduced infection by more than 80% at a concentration of 5 μg/ml in these assays. Some metal chelates of TPPS4 were more active, and a number of sulfonated tetra-aryl derivatives showed significantly higher activity. Some of the most active compounds were the sulfonated tetranaphthyl porphyrin (TNapPS), sulfonated tetra-anthracenyl porphyrin (TAnthPS), and sulfonated 2,6-difluoro-meso-tetraphenylporphine [TPP(2,6-F2)S] and its copper chelate [TPP(2,6-F2)S,Cu], which reduced infection by 99, 96, 94, and 96%, respectively. Our observations indicate that at least some of these compounds are virucidal, i.e., that they render the virus noninfectious. The active compounds were found to inhibit binding of the HIV type 1 gp120 to CD4 and also to completely inhibit the ability of Env proteins expressed from recombinant vectors to induce cell fusion with receptor-bearing target cells. These results support the conclusion that modified porphyrins exhibit substantial activity against HIV and that their target is the HIV Env protein.
The major route of human immunodeficiency virus (HIV) transmission is through sexual contact. Although much emphasis continues to be given to HIV vaccine development, a vaccine capable of preventing HIV type 1 (HIV-1) infection is not yet available. The complexity inherent to the viral life cycle and the added problem of a high mutation rate, which leads to the development of resistance to therapeutic drugs, warrant a greater effort to identify alternative methods to prevent the transmission of HIV. One promising approach is to identify microbicides which, when applied topically, can prevent viral infection. These compounds would directly interact with HIV-1 virions to inactivate infectivity or prevent infection and could be used as an approach to provide a defense against sexual transmission of the virus.
Several previous reports have indicated that certain porphyrins show antiviral activity against HIV infection in assays that measured inhibition of virus replication. Protoporphyrin (3), hemin (22, 25), and other related natural porphyrins and metalloporphyrins (11-13, 16, 25-29) have been shown to have activity against HIV in the micromolar range in such antiviral assays. Sulfonated derivatives of tetraphenylporphyrin have also been shown to be active (16, 28, 38) as have other selected tetra-arylporphyrins (11, 15, 16, 26-28). The mechanisms of action and the stage in the viral life cycle at which the porphyrins exert their inhibitory effects on HIV-1 are not well understood. The effect may be due, at least in part, to inhibition of the reverse transcriptase (RT); various metalloporphyrins in the natural porphyrin class showed significant inhibition of RT activity (41). More-recent work by Argyris and coworkers has identified the connection subdomain of the RT as the site of activity of heme (1, 2). A second mode of action of porphyrins may be inhibition of the HIV protease. Natural porphyrins bearing carborane esters at the 2 and 4 positions have been shown to inhibit the proteases of both HIV-1 and HIV-2 (13). A third mechanism by which porphyrins may inhibit HIV is via interaction with the envelope (Env) protein. Binding to a sequence in the V3 loop of the HIV-1 Env protein has been reported as a possible mechanism by which such compounds could inactivate viral infectivity (12, 25-27, 38). The V3 loop is a principal antigenic determinant of the external domain of the viral Env protein and is thought to play an important role in viral attachment and penetration (6, 36, 44, 47, 50). In the V3 loop at least three positively charged and several hydrophobic amino acids are highly conserved at fixed positions. Debnath et al. (11) hypothesized that porphyrins containing anionic groups may interact with some of the conserved positively charged sites of the V3 loop, which may induce conformational changes in gp120 leading to the inhibition of virus entry into susceptible cells. There is also evidence that the V3 loop is involved in the interaction of HIV with the alternative receptor Gal-Cer, which appears to be involved in infection of epithelial cells (8, 18, 49).
While assays of antiviral activity are relevant for identification of potential therapeutic agents, an assay for identification of potential topical microbicides that could be used for prevention of infection should measure inhibition of infection by cell-free virus. In the present study, we have used such an assay to evaluate a number of natural and synthetic porphyrins and determined some of the structural features that are important for their activity. We also provide new information on the effects of the most active compounds on functional activity of the viral Env protein.
MATERIALS AND METHODS
Porphyrins.
Porphyrins were obtained from Frontier Scientific (Logan, Utah) or Midcentury Chemicals (Posen, Ill.). Porphyrin designations are as follows: PP, protoporphyrin IX; MP, mesoporphyrin IX; HP, hematoporphyrin IX; DP, deuteroporphyrin IX; DPSS, deuteroporphyrin IX 2,4-disulfonic acid; DPSSDME, deuteroporphyrin IX 2,4-disulfonic acid dimethyl ester; DPEG, deuteroporphyrin IX 2,4-bis-ethylene glycol; CoproI, coproporphyrin I; TPP, meso-tetraphenylporphine; TPPS4, meso-tetraphenylporphyrin tetrasulfonate; TPPS3, meso-tetraphenylporphyrin trisulfonate; TNapPS, sulfonated 5,10,15,20-tetra-naphthalen-1-yl-porphyrin; TAnthPS, sulfonated 5,10,15,20-tetra-anthracen-9-yl-porphyrin; TMPS, sulfonated tetramesitylporphyrin (2,4,6-trimethyl substitution [2,4,6-tri-Me] on each phenyl ring); TPPC, meso-tetrakis(4-carboxyphenyl)porphyrin. In all other instances, an S at the end of the name indicates that the parent porphyrin was sulfonated. In most cases, these are compounds with different numbers of sulfonates and/or different positions of the sulfonates on the ring. Additional natural porphyrins are as follows: NP1, 2,4-di-Br-DP,Fe; NP2, PP dipropanol; NP3, MP dipropanol; NP4, PP di-beta-Ala amide,Fe; NP5, MP dipropanol,Fe (the metal chelate of NP3). An additional synthetic porphyrin, SP1, is tri(4-sulfonatophenyl)-mono(4-pyridyl)porphyrin. A compound abbreviation followed by a metal refers to the metal chelate of that compound; e.g., TPPS4,Cu is the copper chelate of TPPS4.
Cell lines.
The mouse NIH 3T3 and human HEp-2 cell lines were obtained from the American Type Culture Collection (Manassas, Va.). The recombinant cell lines human MAGI, monkey sMAGI, and mouse 3T3.T4, 3T3.T4.CCR5, and 3T3.T4.CXCR4 and human T-cell lines CEMx174 and HUT78 were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS (National Institutes of Health [NIH], Bethesda, Md.). The human 293T cell line was kindly provided by S. L. Lydy (Emory University, Atlanta, Ga.). NIH 3T3, HEp-2, 3T3.T4, 3T3.T4.CCR5, 3T3.T4.CXCR4, MAGI, sMAGI, and 293T cells were maintained in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal calf serum. HUT78 and CEMx174 cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum.
Viruses and plasmids.
For construction of recombinant vaccinia viruses, plasmids pRB21 and vRB12 were kindly provided by Bernard Moss (NIH) and David Steinhauer (National Institute for Medical Research, London, United Kingdom). The 3′SHIV-89.6 plasmid was obtained from J. Sodroski (Harvard Medical School, Boston, Mass.). Recombinant vaccinia viruses expressing full-length (VV-239env) and truncated (VV-239T) SIVmac239 Env proteins were previously described by Ritter et al. (34), and VVenv1 expressing the BH10 Env protein was described by Owens and Compans (31). A recombinant vaccinia virus encoding a truncated Env protein of HIV-1 89.6 was constructed as follows. The HIV-1 89.6 truncated env gene was obtained by PCR amplification from the HIV-1 89.6 plasmid with the following primers: a 5′ primer introducing an EcoRI site, 5′-GAGAAGAATTCAGTGGCAATGAGAGTGAAGG-3′, and a 3′ primer introducing an NheI site and a premature stop codon after the codon for amino acid 17 in the cytoplasmic domain, 5′-CCTGTCGGCTAGCCTCGATCATGGGAGGAGGGTCTGAAACGATAATG. The PCR product was then digested by EcoRI and NheI and ligated into EcoRI- and NheI-predigested pRB21 as a donor plasmid for vaccinia virus recombination. The recombinant vaccinia virus was obtained by a plaque selection system using recipient vaccinia virus vRB12, described by Blasco and Moss (4). The plasmid pIIIenv3-1, encoding the Env protein of the HXB2 strain of HIV-1, was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS (NIH). The Tat-responsive HIV long terminal repeat in pIIIenv3-1 was used to promote expression of HXB2 Rev and Env. The helper plasmid pCMVtat was kindly provided by Steven Bartz (Fred Hutchinson Cancer Research Center, Seattle, Wash.). Virus-infected H9/HTLV-IIIB NIH 1983 cells were obtained from the AIDS Research and Reference Reagent Program, and the supernatant was used to infect HUT78 cells. HIV-1 IIIB was produced by continued passage of infected HUT78 cells, and virus stock was prepared as described previously (46). To prepare HIV-1 89.6 virus, 293T cells were transfected with p89.6 (from the NIH AIDS Research and Reference Reagent Program). At 48 h posttransfection, DMEM was removed and the cells were washed once in RPMI 1640. Then 2 × 106 CEMx174 cells were added to a plate in 5 ml of RPMI 1640 containing 10% fetal calf serum and cocultured overnight. The following day, CEMx174 cells were removed from virus-producing 293T cells and placed in T-25 flasks for continued passage. SIVmac1A11 virus stock was described previously (46).
Monoclonal antibodies, antisera, and proteins.
SIM.2 and SIM.4 antibodies recognizing human CD4 and recombinant soluble human CD4 were provided by the NIH AIDS Research and Reference Reagent Program. The recombinant IIIB gp120 protein (baculovirus expressed) was obtained from Intracel (Cambridge, Mass.). Anti-mouse immunoglobulin G peroxidase conjugate was obtained from Sigma (St. Louis, Mo.).
Screening of the porphyrins' ability to prevent HIV infection.
Porphyrin stock solutions were prepared at concentrations of 5 mg/ml, diluted 100-fold in growth medium, and mixed with virus stock. Samples were left in the dark at room temperature for 1 h. For MAGI and sMAGI assays, 25 μl of virus-compound mixture was mixed with 225 μl of growth medium containing DEAE-dextran (15 μg/ml) and 50 μl was added to wells with confluent monolayers of MAGI or sMAGI cells (on a 96-well plate). At 2 h postinfection, an additional 200 μl of complete DMEM was added. After 3 days virucidal activity was measured by removal of the media, fixation with 1% formaldehyde and 0.2% glutaraldehyde, and staining with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). We observed about 50 to 60 separate blue nuclei per well for the positive control. Scoring of blue nuclei in a 96-well format was greatly enhanced by using a planar lens (Olympus; ×4) to visualize the entire well. For determining virus titers we used RT (Roche), MAGI (20), and sMAGI (5) assays. Comparison of the number of blue cells in wells infected with compound-treated virus to the number found in wells infected with untreated virus was used to determine residual viral infectivity (expressed as a percentage). Numerical data reported are the averages of three experiments, each run in duplicate.
Procedure for removal of unbound porphyrin.
Filtration was used to separate free compounds from the virus. Initial tests were performed on a large scale (without virus) so that the concentration of porphyrin could be measured spectroscopically (1601 spectrometer; Cary). Stock solutions of the porphyrin (5 mg/ml) were diluted 100-fold with medium. This solution was in turn diluted 50-fold with Dulbecco's phosphate-buffered saline (PBS). This solution (9 ml) was placed in a filtration apparatus (Centriplus YM-100; 100,000 MWCO; Millipore, Bedford, Mass.) and centrifuged. After three serial filtrations, the experimental concentration was compared to that expected on the basis of simple dilution calculations. For TPPC, with carboxylic acid groups on each of the porphyrin phenyl rings, three serial filtrations-dilutions left about a factor of two more porphyrin in solution than expected from simple dilution calculations. A similar experiment was run with TPPS4,Cu. This sulfonated porphyrin did not pass through the membrane as readily. In this case, the three serial filtrations-dilutions left about a factor of 35 more porphyrin than expected from simple dilution calculations.
In the corresponding biological experiments, 50 μl of the virus-compound mixture was mixed with 450 μl of PBS and loaded into a reservoir with a filter (Microcon YM-100; Millipore Corporation). The sample reservoir was placed into an Eppendorf tube and spun at 10,000 rpm for 3 min. To collect the sample, the reservoir was inverted into a new Eppendorf tube and spun again (recovery spin). The volume of the sample after the recovery spin (about 50 μl) was readjusted to 500 μl with PBS, and the reservoir was spun with a new filter. The procedure was repeated a total of four times. Mathematically, this should have resulted in a 1,000-fold dilution of the porphyrin. From the control experiments, we conclude that the actual dilution was probably about 500-fold for nonsulfonated porphyrins and about 30-fold for sulfonated porphyrins. The final volume was adjusted up to 100 μl with PBS. To this was added 100 μl of 2× DMEM containing 20% fetal bovine serum and 30 μg of DEAE-dextran/ml; 50 μl of the resulting solution was added to the MAGI cells. Controls were tested similarly.
gp120-CD4 binding assay.
To investigate the possible effect of porphyrins on the binding of HIV-1 IIIB gp120 to CD4, we developed a gp120-CD4 binding assay. The assay was developed by modifying that associated with a capture gp120 enzyme-linked immunosorbent assay kit (Intracel Corporation). Briefly, a 96-well plate was coated with soluble CD4 and 0.5 μg of HIV-1 IIIB gp120 per well and was incubated in the presence or absence of test compounds for 1 h at room temperature. After four washes with buffer to remove unbound proteins, the bound gp120 was detected by anti-gp120 peroxidase-conjugated antibodies and quantitated by the protocol provided by the manufacturer.
Cell fusion assays.
For cell fusion assays we compared three different expression systems: (i) a recombinant vaccinia virus expression system which is able to express high levels of Env, (ii) a plasmid expression system which is able to express Env proteins in the absence of other HIV proteins or vaccinia virus proteins, and (iii) cells persistently infected with HIV-1 IIIB or HIV-1 89.6. For recombinant vaccinia viruses expressing HIV-1 Env proteins, HEp-2 cells were infected at a multiplicity of infection of 5. After 24 h cells were collected and counted, and about 2.5 × 103 cells were added to 3T3.T4.CXCR4 or 3T3.T4.CCR5 cell monolayers in 96-well plates in 100 μl of medium in the presence or absence of the test compounds.
For the second assay we transfected 293T cells by the calcium phosphate precipitation method with the plasmid pIIIenv3-1 expressing the HIV-1 Env protein (HXB2 Env) with a long terminal repeat promoter and cotransfected the cells with helper plasmid pCMVTAT at a ratio of 10:1 or with plasmids expressing simian immunodeficiency virus Env proteins by using a cytomegalovirus promoter as described above. After 48 h cells were collected and cocultured with uninfected cells as in the previous assay.
As a third system we used HUT78 cells persistently infected with HIV-1 IIIB or CEMx174 cells persistently infected with HIV-1 89.6. The infected cells were counted and cocultured with uninfected cells as in the previous assays.
For all fusion assays, after 5 or 20 h of cocultivation, the level of cell fusion induced by the untreated recombinant or virus-infected cells and the extent of fusion inhibition by the test compounds were evaluated by microscopic observation. Fusion activities were determined by counting the nuclei in syncytia and comparing the resulting number with the total number of nuclei.
Cytotoxicity test.
A trypan blue exclusion test (42) was used. Compounds at a concentration of 50 μg/ml in growth medium were added to a 96-well plate with MAGI cells. After 72 h cells were detached by a 0.25% trypsin-0.05% EDTA solution and diluted 1:10 in growth medium. To test cell viability, we mixed 1 part of 0.4% trypan blue and 9 parts of diluted cells, incubated the mixture for about 2 min at room temperature, and applied a drop of the trypan blue-cell mixture to a hemacytometer. Using a binocular microscope we then counted stained (nonviable) and unstained (viable) cells. The fraction of viable cells was calculated as the number of unstained cells in the wells treated with compound as a percentage of the number in control wells.
Therapeutic indices.
The 50% cytotoxic concentration (CC50) was defined as the concentration of compound that reduced the viability of cells by 50% (calculated from four different concentrations of porphyrin). The concentration achieving 50% protection was defined as the 50% effective concentration (EC50). The selective index value was defined as the CC50/EC50 ratio.
RESULTS
Anti-HIV activity of porphyrins.
We evaluated the abilities of a series of natural and synthetic compounds (Fig. 1) to inactivate the infectivity of HIV-1 IIIB virus by using a MAGI cell assay. For structure-activity analysis, we divided these porphyrins into three classes: (i) natural porphyrins, (ii) metallo-TPPS4 derivatives, and (iii) sulfonated tetra-arylporphyrins. Each of these classes is discussed below.
FIG. 1.
Structures of porphyrins studied.
Natural porphyrins.
Initially, we tested porphyrins related to protoporphyrin and its iron conjugate, hemin. The protoporphyrin ring skeleton has vinyl groups at the 2 and 4 positions on the periphery of the ring (PP, Fe, Mn, and Zn) (Fig. 1). Other related structures tested involved the replacement of the vinyl groups on the heme periphery at the 2 and 4 positions (metal chelates are in parentheses): MP (Cu and Mn), DP (Co, Cu, Fe, Mn, and Zn), HP (Co, Cu, Mn, and Zn), DPEG (Fe and Zn), DPSS and DPSSDME (Co, Cu, Fe, and Zn), NP1, NP2, and NP3. NP2 and NP3 proved to be toxic. The tetracarboxylic acid CoproI,Fe was tested as well. In general, only compounds with more than 80% inhibition of HIV infection under our assay conditions were studied in more detail. The natural porphyrins did not meet this criterion.
Some studies of porphyrin inhibition of viruses involve photoexcitation of a diamagnetic porphyrin, resulting in the production of singlet oxygen or free radicals or both, which are the agents that damage the viruses (24, 30). Photoactivation was not significant in the present study. In particular, diamagnetic derivatives (which are photoactive) were not in general more active than paramagnetic derivatives (which are not photoactive); e.g., the Fe(III) (paramagnetic), Mn(II) (paramagnetic), and Zn(II) (diamagnetic) derivatives of protoporphyrin gave 80, 65, and 52% inhibition, respectively, indicating that photoactivation does not play a significant role in viral inactivation.
Metallo-TPPS4 derivatives.
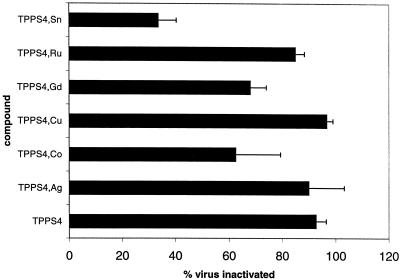
A series of metalloderivatives of TPPS4 was evaluated (Fig. 2). This series has the advantage that each porphyrin has a unique structure, e.g., that all sulfonates are in the 4 position and that each porphyrin has one (and only one) sulfonate on each of the phenyl rings. Metalloderivatives without axial ligands (TPPS4 and its Cu chelate produced 93 and 97% inhibition, respectively) were more effective in preventing infection than derivatives with axial ligands (the Sn, Co, and Gd chelates produced 44, 63, and 68% inhibition, respectively). This relationship may indicate that axial ligands have undesirable steric interactions with the biological target. Some TPPS4 derivatives stack significantly in solution (9, 32, 35). To determine whether the monomeric form of the porphyrin was important for the activity, the self-stacking of these derivatives was evaluated by measuring the optical spectrum of each of the metallo-TPPS4 derivatives as a function of added NaCl. Previous work has shown that this measurement gives data allowing a good estimate of the relative ease of porphyrin stacking (17). The TPPS derivatives evaluated stacked the order TPPS ≈ Ni ≈ Pd > Cu > VO > Mn, Sn > Co, Gd, Ru, TiO (data not shown). There was a general correlation between the propensity to self-stack in solution and the ability of these TPPS4 chelates to block infection by HIV; the derivatives which self-stack were more active in blocking HIV infection. Because self-stacking is greater for derivatives without axial ligands (no metal [e.g., Cu and Ni]), the effect may be due to the enhanced binding of planar species at the biological site rather than stacking per se. As observed for the natural porphyrins, there was no correlation between anti-HIV activity and the paramagnetic or diamagnetic nature of the central metal, indicating that photoactivation does not play a role in virus inhibition.
FIG. 2.
Activity of metallo-TPPS4 against HIV-1 IIIB. Porphyrins at a concentration of 50 μg/ml were incubated with HIV-1 IIIB in the dark for 1 h, diluted 10-fold, and used to inoculate MAGI cells. After 3 days activity against HIV was measured by removal of the media, fixation, and staining with X-Gal. The nuclei of infected cells were stained blue after incubation with X-Gal. The residual HIV infectivity (percent) was measured by dividing the number of blue cells in wells infected with compound-treated virus by the number in wells infected with untreated virus. Data are reported as the means of three independent assays, each run in duplicate. Error bars represent standard deviations.
Sulfonated derivatives of TPP and related porphyrins.
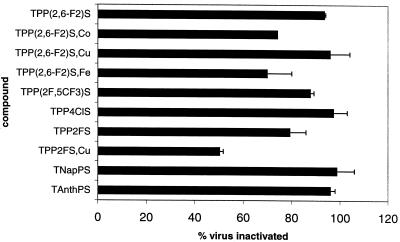
Sulfonated derivatives of TPP and related porphyrins are synthesized by sulfonation of the parent tetra-aryl porphyrin. All are mixtures of compounds including members with different extents of sulfonation and perhaps different positions of the sulfonate on the ring (43). Starting materials included TPP derivatives with 2-, 3-, and 4-chloro substituents as well as the 2- and 4-fluoro substituents. More sterically hindered derivatives had 2,4,6-tri-Me, 2,6-di-F, and 2F, 5CF3 substitution. The sulfonated naphthyl and anthracenyl porphyrins were also studied. Compounds giving greater than 80% inhibition of viral infectivity in initial screens were evaluated in more detail (Fig. 3). The five most active compounds were TNapPS, TAnthPS, sulfonated 2,6-difluoro-meso-tetraphenylporphine [TPP(2,6-F2)S] and its copper chelate TPP(2,6-F2)S,Cu, and TPP4ClS, a sulfonated TPP with one chlorine at the 4-position on each phenyl ring. All of these except the TPP4ClS have substantial steric bulk above and below the plane of the porphyrin. Thus, the results suggest that substitution above and below the plane of the porphyrin may enhance the activity of these species.
FIG. 3.
Activity of sulfonated tetra-arylporphyrins against HIV-1 IIIB. Assays were performed as described in the legend to Fig. 2.
Effective concentration.
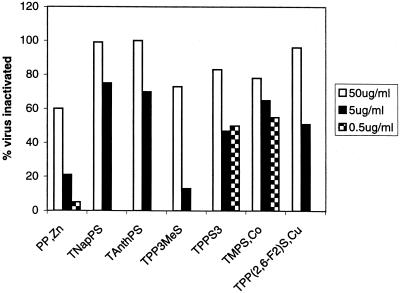
To determine the effective concentration of the compounds, virus samples were mixed with porphyrins at 10-fold dilutions of 50, 5, and 0.5 μg/ml. The most effective concentration was the highest concentration of 50 μg/ml (Fig. 4). However, three compounds also exhibited significant activity at concentrations of 0.5 μg/ml, specifically, TNapPS, TAnthPS, and TPP(2,6-F2)S,Cu. Thus, the most active compounds had an EC50 of less than 5 μg/ml.
FIG. 4.
Concentration dependence of activity. HIV-1 IIIB virus samples were mixed with different concentrations of compounds (50, 5, and 0.5 μg/ml), incubated in the dark for 1 h, diluted 10-fold, and used to inoculate MAGI cells. Residual activity was determined as described for Fig. 2.
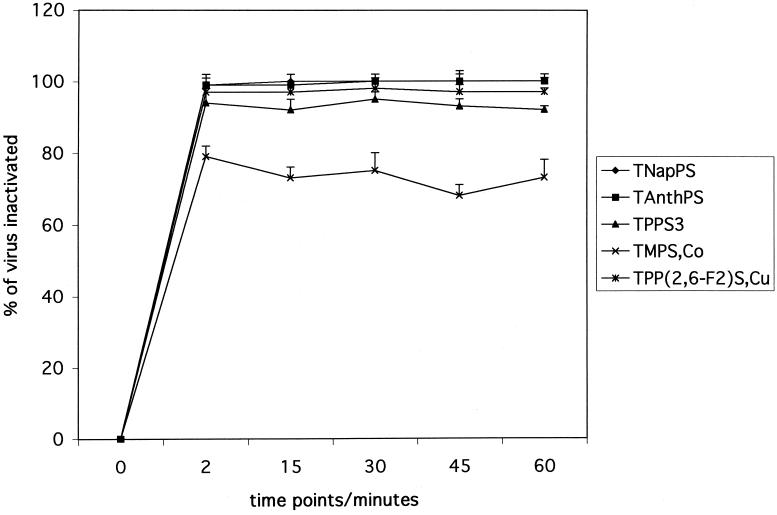
Kinetics of inactivation.
To determine the kinetics of inactivation of viral infectivity, we incubated mixtures of HIV-1 IIIB with five porphyrins at a concentration of 50 μl/ml and assayed residual infectivity at various time intervals (Fig. 5). For all these compounds, the activity observed at 2 min did not change over the time period studied (up to 60 min). This indicates that the interaction of these compounds with HIV-1 IIIB is very rapid and not time dependent. TNapPS and TAnthPS prevented viral infection almost completely in this assay. TPP(2,6-F2)S,Cu was only slightly less active. When we tested these compounds at a concentration of 5 μg/ml, we also found similar levels of inactivation of virus at all time points but generally the inactivation was less complete than that at higher concentrations (data not shown).
FIG. 5.
Kinetics of inactivation of HIV-1 IIIB. Compounds at a concentration of 50 μg/ml were mixed with virus, incubated for various time intervals (0, 15, 30, 45, and 60 min), and diluted 1:10 with complete medium, and infectivity titers were determined as described for Fig. 2.
Virucidal activity of porphyrins.
To determine whether the virus, once treated, was still rendered noninfectious once the unbound compound was removed from the solution, we used a filtration-dilution method. Solutions of the virus and compound were filtered until only about 10% of the original volume remained. The solution that had not gone through the filter was diluted to the original volume, and the process was repeated four times. Spectroscopic assays showed that four dilutions resulted in the original porphyrin concentrations being reduced by 30- to 500-fold (see Materials and Methods). For these filtration assays, we selected compounds in two categories: three active porphyrins [TNapPS, TAnthPS, and TPP(2,6-F2)S,Cu] and two porphyrins with intermediate activity [TMPS,Co and TPP(2,6-F2)S,Fe].
We found that TNapPS and TAnthPS had high anti-HIV activity in the screening assay (without removal of free compound) as well as after removal of compounds by the filtration-dilution method, with about 90 to 99% inactivation of the virus either with or without filtration. This demonstrates that the compounds exhibit virucidal activity, i.e., that the virus has been rendered noninfectious on the time scale of the experiment. TPP(2,6-F2)S,Cu inhibited about 95% of the virus in the screening assay and about 80% of the virus after filtration-dilution. TMPS,Co and TPP(2,6-F2)S,Fe had about 80% anti-HIV activity in the screening assay and about 20 to 40% anti-HIV activity after filtration-dilution. The partial recovery of virus infectivity observed with these compounds may be due to disassociation of the porphyrin from the viral envelope structure during the filtration-dilution.
Activity with other lentiviruses.
To investigate whether the compounds with high activity would inactivate other HIV strains and lentiviruses, we extended our studies to HIV-1 89.6 and SIVmac1A11. We selected the most active compounds against HIV-1 IIIB, TNapPS, TAnthPS, and TPP(2,6-F2)S,Cu, and also used two compounds with intermediate activity, TPPS4,Co and TPPS4,Ag. We found that both viruses were sensitive to the most active compounds, TNapPS, with 76% of 89.6 and 88% of SIVmac1A11 being inactivated, TAnthPS, with 90% of 89.6 and 84% of SIVmac1A11 being inactivated, and TPP(2,6-F2)S,Cu, with 98% of HIV 89.6 and 84% of SIVmac1A11 being inactivated (data not shown). We also observed that the compounds TPPS4,Co and TPPS4,Ag inactivated about 50 to 70% of HIV-1 89.6 infectivity. Thus, the porphyrins with activity against a laboratory-adapted virus (IIIB) were also active against a primary HIV isolate (89.6) as well as against simian immunodeficiency virus.
Toxicity.
We used a trypan blue exclusion test to determine possible toxicity of the test compounds. Compounds at a concentration of 50 μg/ml in growth medium were added to MAGI cells. This concentration is the same as that used for pretreatment of virus; however, it is 10-fold higher than that used when the compounds are applied to MAGI cells for a virus assay. After 72 h, a trypan blue assay was used to compare the viability of cells treated with compounds to that of untreated cells. Of the three most active compounds, TAnthPS did not have any detectable toxic effect. TNapPS and TPP(2,6-F2)S,Cu showed 55 and 60% toxicity, respectively. The most active of the natural porphyrins, DPEG,Fe, also did not have any detectable toxic effect. Three natural porphyrins with no activity were also tested for toxicity. Cells treated with DP,Mn were 100% viable, those treated with DP,Cu were about 71% viable, and those treated with DP,Co were about 59% viable. TPP4ClS, the sulfonated TPP with one halogen with the best activity against HIV, showed about 50% toxicity. TPP3ClS, also a member of this class, was found to be too toxic for accurate measurement of activity of virus inhibition. The most active of the sulfonated TPP derivatives with two halogens, TPP(2,6-F2)S, showed about 50% toxicity. All of these data indicate that there is no correlation of virucidal activity and toxicity. It is encouraging that a number of the most active compounds in each class showed no detectable toxic effect.
Therapeutic indices for three of the most active compounds were determined by measuring both activity and toxicity at four concentrations of porphyrin. TNapPS (EC50 = 5 μg/ml; CC50 = 75 μg/ml), TPP(2,6-F2)S,Cu (EC50 = 5 μg/ml; CC50 = 250 μg/ml), and TPPS3 (EC50 = 5 μg/ml; CC50 = 50 μg/ml) had CC50/EC50 values of 15, 50, and 10, respectively.
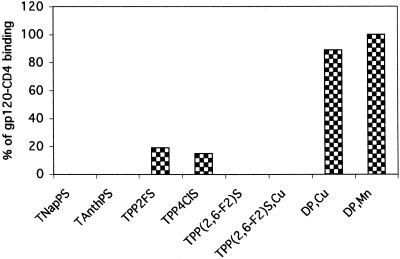
Effect of porphyrins on interaction of gp120 with CD4.
To investigate the site of action of the porphyrins, we initially investigated the effects of the binding of gp120 to its primary receptor, CD4 (10). CD4 binding results in a conformational change in gp120 (37) that enables it to interact with a coreceptor, generally either CCR5 or CXCR4 (21). To investigate the effect of porphyrins on the binding of gp120 to CD4, we used the gp120-CD4 binding assay described in Materials and Methods. We tested the inhibition of binding using three groups of compounds. Four of the porphyrins [TNapPS, TAnthPS, TPP(2,6-F2)S, and TPP(2,6-F2)S,Cu] from the group with the highest activity against HIV were found to completely inhibit the binding of gp120 to CD4 (Fig. 6). TPP4ClS showed about 97% inhibition of HIV and 85% inhibition of gp120-CD4 binding. TPP2FS had about 80% activity against HIV and 81% inhibition of gp120-CD4 binding. A third control group of porphyrins, which did not have significant anti-HIV activity (e.g., DP,Cu and DP,Mn), also did not inhibit binding or had only low activity. A greater effect on binding than on infectivity was observed when we used a gp120-CD4 binding assay to investigate the effective concentration. These results show a general correlation between activity against HIV and inhibition of gp120 binding to CD4, although the latter was found to be more sensitive to inhibition by compounds with intermediate levels of activity against HIV.
FIG. 6.
Inhibition of gp120-CD4 binding. A 96-well plate coated with soluble CD4 was incubated with HIV-1 IIIB gp120 in the presence or absence of compounds for 1 h at room temperature. After extensive washes the bound gp120 was detected by anti-gp120 peroxidase-conjugated antibodies. Results represent percentages of gp120 binding compared to that for untreated gp120 samples (100%).
Inhibition of HIV-induced cell fusion by porphyrins.
To determine if porphyrins had an effect on the functional activity of the Env protein, we investigated their effects by using assays for cell fusion activity (Table 1; Fig. 7). We compared three different expression systems for the Env proteins, which differ with respect to the expression of other encoded proteins.
TABLE 1.
Inhibition of Env-induced cell fusion by porphyrinsa
| Compound | Presence of nuclei in syncytia
|
|||
|---|---|---|---|---|
| pIIIenv X4, 6 h | VVenv1 X4, 6/23 h | VV89.6 envt X4, 6/23 h | VV89.6 envt R5, 6/23 h | |
| None | 1+ | 4+/4+ | 4+/4+ | 4+/4+ |
| TAnthS | − | −/− | −/− | −/− |
| TNapPS | − | −/− | −/− | −/− |
| DP,Cu | 1+ | 4+/4+ | 4+/4+ | 4+/4+ |
| DP,Mn | 1+ | 4+/4+ | 4+/4+ | 4+/4+ |
| DP,Co | 1+ | 4+/4+ | 4+/4+ | 4+/4+ |
| DP,Sn | 1+ | 4+/4+ | 4+/4+ | 4+/4+ |
| TPP2FS | − | −/4+ | 1+/4+ | 4+/4+ |
| TPP(2,6-F2)S | − | −/− | 1+/4+ | 1+/4+ |
| TPP(2,6-F2)S,Cu | − | −/− | −/− | −/− |
| TPP(2F,5CF3)S | − | −/4+ | 2+/4+ | 3+/4+ |
| TPPS4 | − | −/− | −/4+ | 1+/4+ |
| TPPS4,Cu | − | −/− | −/− | −/− |
| TPPS4,Ru | 1+ | −/− | −/4+ | 1+/4+ |
| TPPS4,Pd | − | −/− | −/4+ | −/4+ |
| TPPS4,Ni | 1+ | −/− | −/3+ | −/1+ |
| TPPS4,Mn | − | 2+/4+ | 4+/4+ | 4+/4+ |
| TPPS4,TiO | − | −/− | −/4+ | −/4+ |
| NP1 | − | 4+/4+ | 4+/4+ | 3+/4+ |
| NP2 | 1+ | 4+/4+ | 4+/4+ | 4+/4+ |
| HP,Co | 1+ | 4+/4+ | 4+/4+ | 4+/4+ |
| HP,Cu | − | −/− | 1+/4+ | 1+/2+ |
| HP,Znb | − | −/− | 1+/− | −/− |
| TPP3MeSb | − | −/− | 1+/− | 2+/− |
| TPPS3 | − | −/− | 1+/4+ | −/4+ |
Fusion activities were determined by comparing the nuclei in syncytia to the total nuclei. 4+, more than 50% of nuclei are in syncytia; 3+, 30 to 50% of nuclei are in syncytia; 2+, 30 to 10% of nuclei are in syncytia; +, less than 10% of nuclei are in syncytia; −, no syncytia were observed.
Cells show toxicity at 23 h.
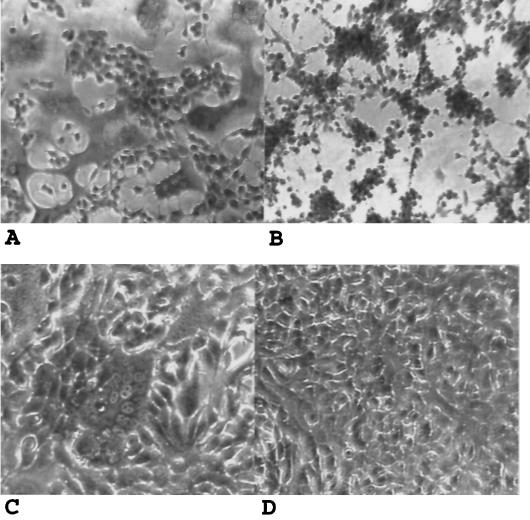
FIG. 7.
Inhibition of fusion by porphyrins. About 2.5 × 103 HEp-2 cells infected with VVenv1, the vaccinia virus recombinant expressing the HIV-1 IIIB Env protein (A and B), or 293T cells transfected with plasmid pIIIenv3-1 (C and D) were added to 3T3.T4.CXCR4 cells in a 96-well plate in the presence or absence of the test compounds and incubated for 5 h for syncytium analysis. Samples were fixed, stained with crystal violet, and photographed under a phase-contrast microscope. (A and C) Untreated samples (without compound); (B and D) samples cocultivated in the presence of TNapPS.
Initially, we used a recombinant vaccinia virus expression system which is able to express high levels of Env. Experiments were run with a recombinant expressing the IIIB Env of HIV-1, which has tropism for the X4 coreceptor (VVenv1), and a recombinant expressing the 89.6 Env, a primary viral isolate with dual tropism for both X4 and R5 coreceptors (VV89.6 envt). We observed complete inhibition of HIV-induced cell fusion with TNapPS, TAnthPS, and TPP(2,6-F2)S,Cu, which had excellent activity against HIV and which completely blocked gp120-CD4 binding. We also observed complete inhibition of fusion in all three assays of cells treated with TPPS4,Cu. This compound had intermediate levels of activity against HIV in the MAGI assay and blocked gp120-CD4 binding about 80 to 100%. We observed that inhibition by these compounds of fusion induced by VVenv1 was more extensive than that of fusion induced by VV89.6 envt (Table 1).
We also used a plasmid expression system that is able to express Env proteins in the absence of other HIV proteins or vaccinia virus proteins (pIIIenv). As with the systems above, we observed complete inhibition of HIV-induced cell fusion with compounds TNapPS, TAnthPS, and TPP(2,6-F2)S,Cu. Many other compounds also exhibited complete inhibition in this assay.
Finally, we also examined fusion activity in cells persistently infected with HIV-1 IIIB or HIV-1 89.6 viruses, which were cocultivated with uninfected target cells in the presence or absence of test compounds. The fusion activity observed in this assay was comparable to that found by using plasmids expressing Env and lower than that observed with Env expressed by vaccinia virus. The results of fusion inhibition by the compounds tested correlated well with those observed by using the other two expression systems (data not shown).
These results demonstrate that the porphyrins with high or intermediate levels of activity against HIV are able to effectively inhibit the membrane fusion activity of the viral Env proteins, a biological function that is important for viral entry as well as the induction of viral cytopathic effects.
DISCUSSION
The central goal of our study was to identify novel porphyrins with activity against HIV that could be useful as topical microbicides to provide a defense against sexual transmission of the virus. The vaginal and gastrointestinal surfaces play a major role in the pathogenesis of infection by HIV-1 as potential routes for viral entry. To determine the activity against HIV of test compounds, we used a MAGI assay that is based on usage of an epithelial cell line. According to our experiments for analyzing kinetics, effective concentration, and fusion inhibition, the most active compounds were TNapPS, TAnthPS, and TPP(2,6-F2)S,Cu. These compounds were also able to inhibit infection by dualtropic HIV-1 89.6 as well as SIVmac1A11 viruses. With TNapPS and TAnthPS, only approximately 1% infected cells remained after a 2-min incubation, indicating a very rapid inactivation.
A major mechanism for activity against HIV may involve porphyrin binding to the V3 loop of gp120. Our results indicate that the porphyrins blocked the binding of gp120 to CD4 and inhibited the cell fusion activity of Env proteins when expressed from recombinant vectors. These results showed that an important target of these compounds is the viral Env protein. Neurath et al. (26, 27) have correlated the anti-HIV activity by using an assay for cytotoxicity on a T-cell line that measures the inhibition of the interaction between gp120 and antibodies specific for the V3 hypervariable loop of this protein. In this series of approximately 20 porphyrins from both the natural and synthetic classes, there was no clear correlation overall between inhibition of antibody binding to gp120 and overall activity in an antiviral assay. However, there was a correlation for the most active members of the series. Debnath et al. have found an excellent correlation between predicted and observed anti-HIV-1 activity by using a 3D QSAR model (11). For a data set composed primarily of natural porphyrins and TPPC derivatives, they observed that the active site apparently is best accommodated by a porphyrin bearing three negatively charged substituents and groups which can provide positive van der Waals interactions at positions corresponding to the 2 and 4 positions of protoporphyrin.
We observed that porphyrins were able to inhibit the cell fusion activity of the HIV Env protein. To exclude the possibility that such an inhibitory effect could be due to an indirect effect, we demonstrated that cell fusion induced by recombinant vectors in the absence of any other HIV protein was also sensitive to inhibition by porphyrins. These results provide strong evidence that the porphyrins are able to effectively inhibit an important function of the Env protein that is needed for viral entry. Song et al. have also correlated anti-HIV activity with syncytium inhibition for a series of synthetic anionic porphyrins and metalloporphyrins (38). No clear overall correlation was seen, but compounds with an EC50 versus HIV of <10 μg/ml all had EC50 values for syncytium inhibition of <40 μg/ml.
Currently several categories of compounds are undergoing thorough testing as potential microbicides to prevent HIV transmission. The first agents to be tested extensively were surface-disruptive agents (surfactants and detergents) that kill or inactivate viruses (vaginal virucides) such as nonoxynol-9. Unfortunately, this class of compounds causes damage to human tissues, leading to inflammation and ulceration (40). After extensive testing it was also determined that the use of this surfactant actually increases the risk of acquiring HIV infection during sexual transmission (19, 33, 45), and its development as an agent to prevent HIV infection has therefore been discontinued. A second group of compounds includes peptides and antibodies which enhance the normal vaginal defense mechanisms (23, 48). A possible limitation of such compounds is the difficulty of their formulation for use as vaginal microbicides. A third group includes nonspecific enhancers of normal vaginal defense mechanisms (lactobacilli, acid buffers, and peroxidases) (7). These compounds did not fully inactivate individual virus particles that are potentially capable of infection at sites of injury. A fourth group includes polymers such as Carraguard (39). This vaginal microbicide gel containing the red seaweed extract carrageenan has been shown to block HIV and other sexually transmitted agents in vitro. However, such polymers may not be fully protective because of possible escape of some virus particles from interaction with the macromolecules. The sulfonated porphyrins are polyanionic molecules. They inhibit viral binding to, fusion with, and entry into susceptible cells, as do some other polyanionic species, including polymers (14). Porphyrins, however, are relatively small molecules and are convenient for formulation into vaginal gels. Their interaction with the virus appears to be very rapid. For some of the molecules studied, removal of free compound did not result in significant recovery of infectivity, indicating that they are effective virucidal agents. For other molecules, removal of free compound results in partial recovery of infectivity, possibly due to their dissociation from target sites on surfaces of virions. However, this is not an important concern for their use as microbicides, because the compounds will continue to be present at sites of transmission during exposure to virus in vivo. Therefore, porphyrins represent a promising class of compounds for further development as microbicides to prevent HIV transmission.
Acknowledgments
This study was supported by NIH grant AI45883.
We thank Atia Alam, Tracy Tie, and Dahnide Taylor for technical assistance and Tanya Cassingham for assistance in preparing the manuscript.
REFERENCES
- 1.Argyris, E. G., J. M. Vanderkooi, and Y. Paterson. 2001. Mutagenesis of key residues identifies the connection subdomain of HIV-1 reverse transcriptase as the site of inhibition by heme. Eur. J. Biochem. 268:925-931. [DOI] [PubMed] [Google Scholar]
- 2.Argyris, E. G., J. M. Vanderkooi, P. S. Venkateswaran, B. K. Kay, and Y. Paterson. 1999. The connection domain is implicated in metalloporphyrin binding and inhibition of HIV reverse transcriptase. J. Biol. Chem. 274:1549-1556. [DOI] [PubMed] [Google Scholar]
- 3.Asanaka, M., T. Kurimura, H. Toya, J. Ogaki, and Y. Kato. 1989. Anti-HIV activity of protoporphyrin. AIDS 3:403-404. [DOI] [PubMed] [Google Scholar]
- 4.Blasco, R., and B. Moss. 1995. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene 158:157-162. [DOI] [PubMed] [Google Scholar]
- 5.Chackerian, B., N. L. Haigwood, and J. Overbaugh. 1995. Characterization of a CD4-expressing macaque cell line that can detect virus after a single replication cycle and can be infected by diverse simian immunodeficiency virus isolates. Virology 213:386-394. [DOI] [PubMed] [Google Scholar]
- 6.Chan, S. Y., R. F. Speck, C. Power, S. L. Gaffen, B. Chesebro, and M. A. Goldsmith. 1999. V3 recombinants indicate a central role for CCR5 as a coreceptor in tissue infection by human immunodeficiency virus type 1. J. Virol. 73:2350-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke, J. G., J. F. Peipert, S. L. Hillier, W. Heber, L. Boardman, T. R. Moench, and K. Mayer. 2002. Microflora changes with the use of a vaginal microbicide. Sex. Transm. Dis. 29:288-293. [DOI] [PubMed] [Google Scholar]
- 8.Cook, D. G., J. Fantini, S. L. Spitalnik, and F. Gonzalez-Scarano. 1994. Binding of human immunodeficiency virus type I (HIV-1) gp120 to galactosylceramide (GalCer): relationship to the V3 loop. Virology 201:206-214. [DOI] [PubMed] [Google Scholar]
- 9.Corsini, A., and O. Herrmann. 1986. Aggregation of meso-tetra(p-sulphonatophenyl)porphine and its Cu(II) and Zn(II) complexes in aqueous solution. Talanta 33:335-339. [DOI] [PubMed] [Google Scholar]
- 10.Dalgleish, A. G., P. C. Beverley, P. R. Clapham, D. H. Crawford, M. F. Greaves, and R. A. Weiss. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763-767. [DOI] [PubMed] [Google Scholar]
- 11.Debnath, A. K., S. Jiang, N. Strick, K. Lin, P. Haberfield, and A. R. Neurath. 1994. Three-dimensional structure-activity analysis of a series of porphyrin derivatives with anti-HIV-1 activity targeted to the V3 loop of the gp120 envelope glycoprotein of the human immunodeficiency virus type 1. J. Med. Chem. 37:1099-1108. [DOI] [PubMed] [Google Scholar]
- 12.Debnath, A. K., S. B. Jiang, N. Strick, K. Lin, S. B. Kahl, and A. R. Neurath. 1999. Anti-HIV-1 activity of carborane derivatives of porphyrins. Med. Chem. Res. 9:267-275. [Google Scholar]
- 13.DeCamp, D. L., L. M. Babé, R. Salto, J. L. Lucich, M.-S. Koo, S. B. Kahl, and C. S. Craik. 1992. Specific inhibition of HIV-1 protease by boronated porphyrins. J. Med. Chem. 35:3426-3428. [DOI] [PubMed] [Google Scholar]
- 14.De Clercq, E. 2002. Strategies in the design of antiviral drugs. Nat. Rev. Drug Discov. 1:13-25. [DOI] [PubMed] [Google Scholar]
- 15.Dixon, D. W., M. S. Kim, V. Kumar, G. Obara, L. G. Marzilli, and R. F. Schinazi. 1992. Amino- and hydroxytetraphenylporphyrins with activity against the human immunodeficiency virus. Antivir. Chem. Chemother. 3:279-282. [Google Scholar]
- 16.Dixon, D. W., L. G. Marzilli, and R. F. Schinazi. 1990. Porphyrins as agents against the human immunodeficiency virus. Ann. N. Y. Acad. Sci. 616:511-513. [Google Scholar]
- 17.Dixon, D. W., and V. Steullet. 1998. Dimerization of tetracationic porphyrins: ionic strength dependence. J. Inorg. Biochem. 69:25-32. [DOI] [PubMed] [Google Scholar]
- 18.Fantini, J., D. G. Cook, N. Nathanson, S. L. Spitalnik, and F. Gonzalez-Scarano. 1993. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus is associated with cell surface expression of galactosylceramide, a potential alternative gp120 receptor. Proc. Natl. Acad. Sci. USA 90:2700-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 20.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapham, C. K., J. Ouyang, B. Chandrasekhar, N. Y. Nguyen, D. S. Dimitrov, and H. Golding. 1996. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science 274:602-605. [DOI] [PubMed] [Google Scholar]
- 22.Levere, R. D., Y.-F. Gong, A. Kappas, D. J. Bucher, G. P. Wormser, and N. G. Abraham. 1991. Heme inhibits human immunodeficiency virus 1 replication in cell cultures and enhances the antiviral effect of zidovudine. Proc. Natl. Acad. Sci. USA 88:1756-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascola, J. R. 2002. Passive transfer studies to elucidate the role of antibody-mediated protection against HIV-1. Vaccine 20:1922-1925. [DOI] [PubMed] [Google Scholar]
- 24.Matthews, J. L., F. Sogandares-Bernal, M. Judy, K. Gulliya, J. Newman, T. Chanh, and A. J. Marengo-Rowe. 1992. Inactivation of viruses with photoactive compounds. Blood Cells 18:75-88. [PubMed] [Google Scholar]
- 25.Neurath, A. R., P. Haberfield, B. Joshi, I. K. Hewlett, N. Strick, and S. Jiang. 1991. Rapid prescreening for antiviral agents against HIV-1 based on their inhibitory activity in site-directed immunoassays. I. The V3 loop of gp120 as target. Antivir. Chem. Chemother. 2:303-312. [Google Scholar]
- 26.Neurath, A. R., N. Strick, and A. K. Debnath. 1995. Structural requirements for and consequences of an antiviral porphyrin binding to the V3 loop of the human immunodeficiency virus (HIV-1) envelope glycoprotein gp120. J. Mol. Recognit. 8:345-357. [DOI] [PubMed] [Google Scholar]
- 27.Neurath, A. R., N. Strick, P. Haberfield, and S. Jiang. 1992. Rapid prescreening for antiviral agents against HIV-1 based on their inhibitory activity in site-directed immunoassays. II. Porphyrins reacting with the V3 loop of gp120. Antivir. Chem. Chemother. 3:55-63. [Google Scholar]
- 28.Neurath, A. R., N. Strick, and S. Jiang. 1994. Rapid prescreening for antiviral agents against HIV-1 based on their inhibitory activity in site-directed immunoassays. Approaches applicable to epidemic HIV-1 strains. Antivir. Chem. Chemother. 4:207-214. [Google Scholar]
- 29.Neurath, A. R., N. Strick, K. Lin, A. K. Debnath, and S. Jiang. 1994. Tin protoporphyrin-IX used in control of heme metabolism in humans effectively inhibits HIV-1 infection. Antivir. Chem. Chemother. 5:322-330. [Google Scholar]
- 30.North, J., H. Neyndorff, and J. G. Levy. 1993. Photosensitizers as virucidal agents. J. Photochem. Photobiol. B Biol. 17:99-108. [DOI] [PubMed] [Google Scholar]
- 31.Owens, R. J., and R. W. Compans. 1989. Expression of the human immunodeficiency virus envelope glycoprotein is restricted to basolateral surfaces of polarized epithelial cells. J. Virol. 63:978-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasternack, R. F., P. R. Huber, P. Boyd, G. Engasser, L. Francesconi, E. J. Gibbs, P. Fasella, G. C. Venturo, and L. D. C. Hinds. 1972. On the aggregation of meso-substituted water-soluble porphyrins. J. Am. Chem. Soc. 94:4511-4517. [DOI] [PubMed] [Google Scholar]
- 33.Richardson, B. A., L. Lavreys, H. L. Martin, Jr., C. E. Stevens, E. Ngugi, K. Mandaliya, J. Bwayo, J. Ndinya-Achola, and J. K. Kreiss. 2001. Evaluation of a low-dose nonoxynol-9 gel for the prevention of sexually transmitted diseases: a randomized clinical trial. Sex. Transm. Dis. 28:394-400. [DOI] [PubMed] [Google Scholar]
- 34.Ritter, G. D., Jr., M. J. Mulligan, S. L. Lydy, and R. W. Compans. 1993. Cell fusion activity of the simian immunodeficiency virus envelope protein is modulated by the intracytoplasmic domain. Virology 197:255-264. [DOI] [PubMed] [Google Scholar]
- 35.Rubires, R., J. Crusats, Z. El-Hachemi, T. Jaramillo, M. Lopez, E. Valls, J. A. Farrera, and J. M. Ribo. 1999. Self-assembly in water of the sodium salts of meso-sulfonatophenyl substituted porphyrins. New J. Chem. 23:189-198. [Google Scholar]
- 36.Sakaida, H., T. Hori, A. Yonezawa, A. Sato, Y. Isaka, O. Yoshie, T. Hattori, and T. Uchiyama. 1998. T-tropic human immunodeficiency virus type 1 (HIV-1)-derived V3 loop peptides directly bind to CXCR-4 and inhibit T-tropic HIV-1 infection. J. Virol. 72:9763-9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sattentau, Q. J., and J. P. Moore. 1991. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J. Exp. Med. 174:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song, R., M. Witvrouw, D. Schols, A. Robert, J. Balzarini, E. De Clercq, J. Bernadou, and B. Meunier. 1997. Anti-HIV activities of anionic metalloporphyrins and related compounds. Antivir. Chem. Chemother. 8:85-97. [Google Scholar]
- 39.Spieler, R. 2002. Seaweed compound's anti-HIV efficacy will be tested in southern Africa. Lancet 359:1675. [DOI] [PubMed] [Google Scholar]
- 40.Stafford, M. K., H. Ward, A. Flanagan, I. J. Rosenstein, D. Taylor-Robinson, J. R. Smith, J. Weber, and V. S. Kitchen. 1998. Safety study of nonoxynol-9 as a vaginal microbicide: Evidence of adverse effects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:327-331. [DOI] [PubMed] [Google Scholar]
- 41.Staudinger, R., N. G. Abraham, R. D. Levere, and A. Kappas. 1996. Inhibition of human immunodeficiency virus-1 reverse transcriptase by heme and synthetic heme analogs. Proc. Assoc. Am. Physicians 108:47-54. [PubMed] [Google Scholar]
- 42.Strober, W. 1994. Trypan blue exclusion test of cell viability, p. A.3.3-A.3.4. In J. E. Coligan and A. M. Kruisbeek (ed.), Current protocols in immunology. Wiley-Greene, New York, N.Y.
- 43.Sutter, T. P. G., R. Rahimi, P. Hambright, J. C. Bommer, M. Kumar, and P. Neta. 1993. Steric and inductive effect on the basicity of porphyrins and on the site of protonation of porphyrin dianions. J. Chem. Soc. Faraday Trans. 89:495-502. [Google Scholar]
- 44.Vallejo, A., A. Heredia, A. Mas, S. F. Lee, J. S. Epstein, V. Soriano, and I. K. Hewlett. 1998. Tropism, coreceptor use, and phylogenetic analysis of both the V3 loop and the protease gene of three novel HIV-1 group O isolates. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18:417-425. [DOI] [PubMed] [Google Scholar]
- 45.van de Wijgert, J., and C. Coggins. 2002. Microbicides to prevent heterosexual transmission of HIV: ten years down the road. BETA 15:23-28. [PubMed] [Google Scholar]
- 46.Vzorov, A. N., and R. W. Compans. 2000. Effect of the cytoplasmic domain of the simian immunodeficiency virus envelope protein on incorporation of heterologous envelope proteins and sensitivity to neutralization. J. Virol. 74:8219-8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, W. K., T. Dudek, M. Essex, and T. H. Lee. 1999. Hypervariable region 3 residues of HIV type 1 gp120 involved in CCR5 coreceptor utilization: therapeutic and prophylactic implications. Proc. Natl. Acad. Sci. USA 96:4558-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber, J., A. Nunn, T. O'Connor, D. Jeffries, V. Kitchen, S. McCormack, J. Stott, N. Almond, A. Stone, and J. Darbyshire. 2001. ‘Chemical condoms’ for the prevention of HIV infection: evaluation of novel agents against SHIV(89.6PD) in vitro and in vivo. AIDS 15:1563-1568. [DOI] [PubMed] [Google Scholar]
- 49.Yahi, N., S. Baghdiguian, and J. Fantini. 1995. Production of a highly cytopathic HIV-1 isolate from a human mucosal epithelial cell line cultured on microcarrier beads in serum-free medium. In Vitro Cell. Dev. Biol. Anim. 31:62-66. [DOI] [PubMed] [Google Scholar]
- 50.Zwart, G., H. Langedijk, L. van der Hoek, J. J. de Jong, T. F. Wolfs, C. Ramautarsing, M. Bakker, A. de Ronde, and J. Goudsmit. 1991. Immunodominance and antigenic variation of the principal neutralization domain of HIV-1. Virology 181:481-489. [DOI] [PubMed] [Google Scholar]