Abstract
Light-scattering intensities in the I parallel and I+ mode were obtained on thin sections of three human lenses. Random density and orientation fluctuation theory, without cross correlation, was employed to evaluate light-scattering parameters. Both the density correlation distances, as well as the orientation correlation distances, were related to structural elements in the lens fiber cell that have been observed by other investigators with different techniques. The magnitude of these fluctuations were evaluated, and it was demonstrated that the density fluctuations are the main contributors to light scattering in normal human lenses. Changes in the light-scattering parameters were evaluated as a function of position within the lens. The changes observed agree with the biochemical data in the literature that reflects that an aging process occurs when one proceeds from the periphery of the lens toward the center.
Full text
PDF
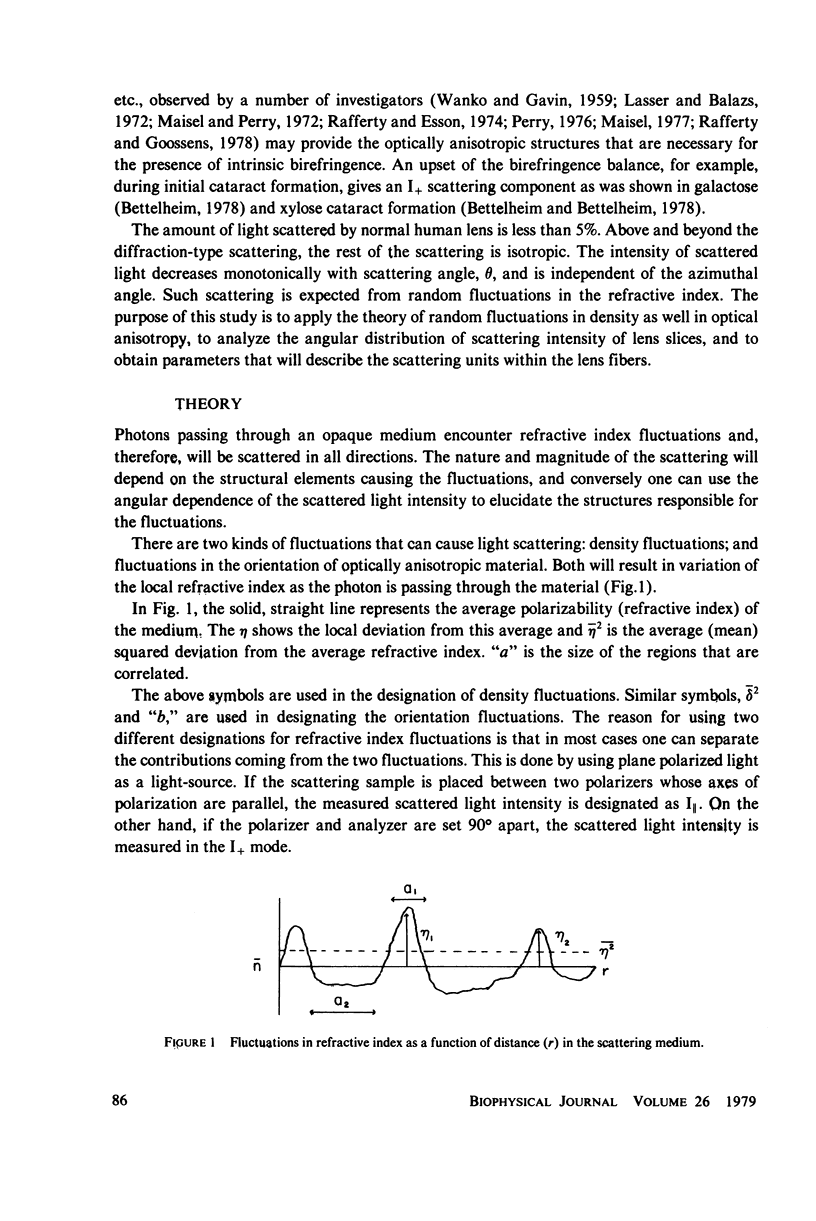



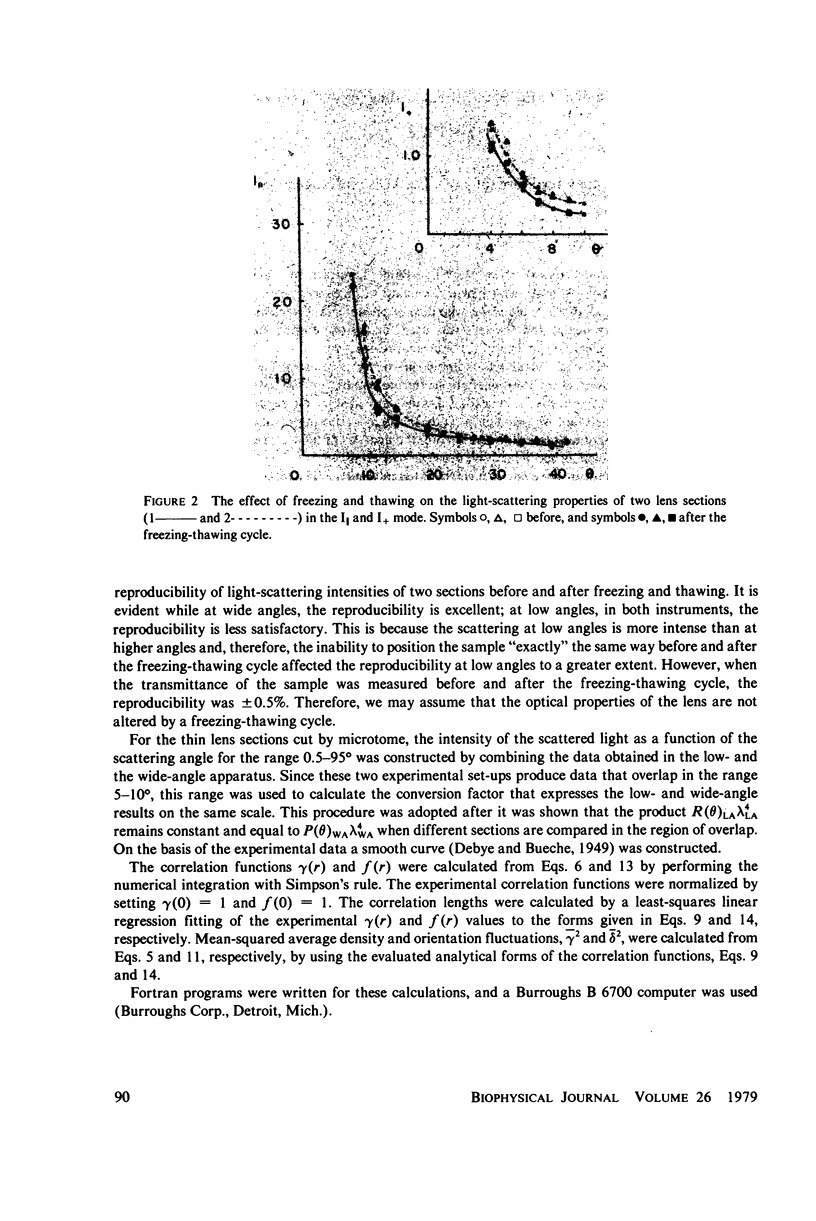
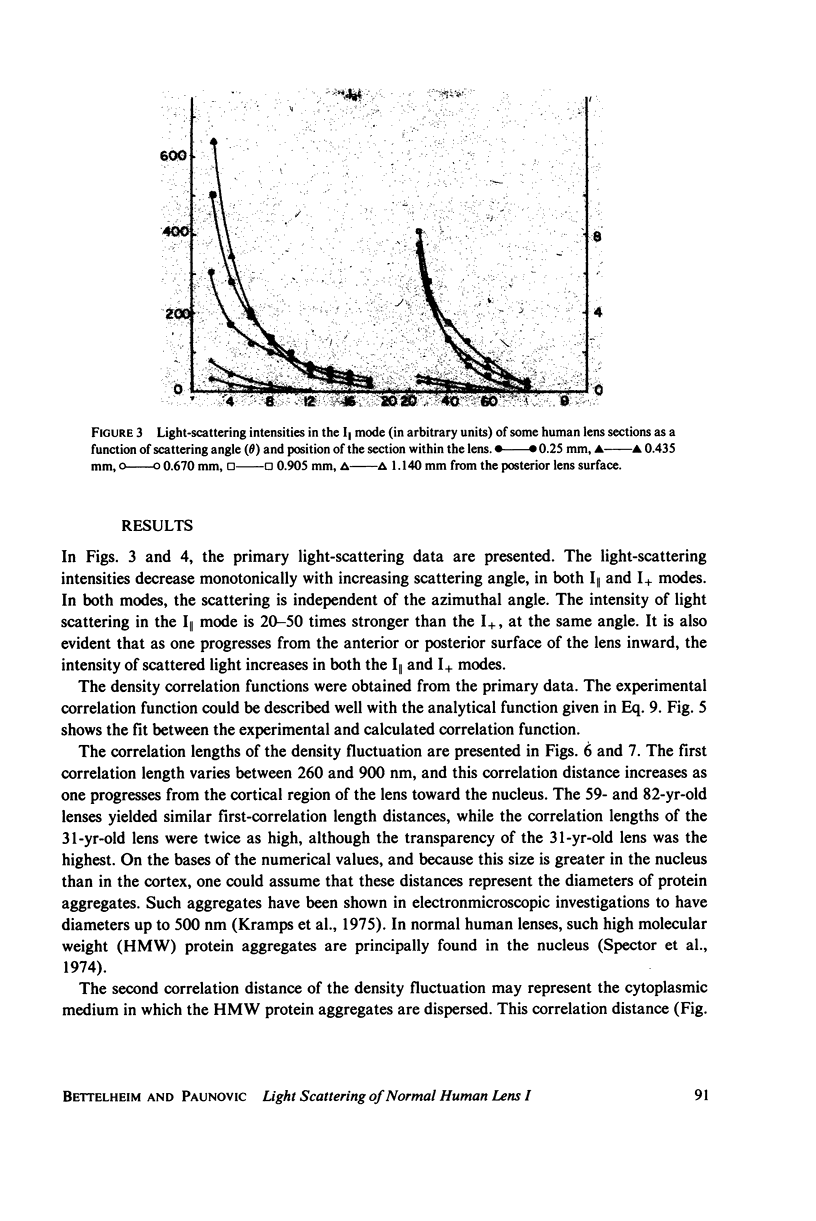
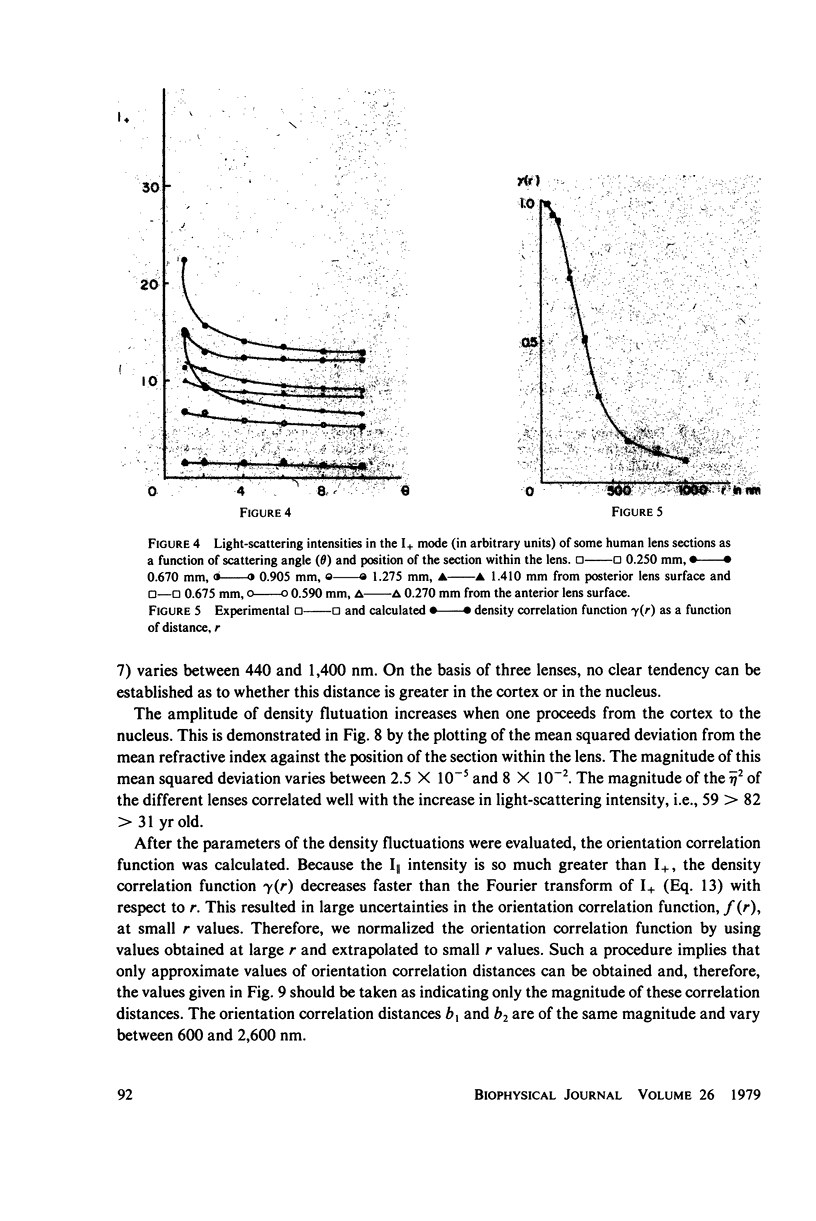
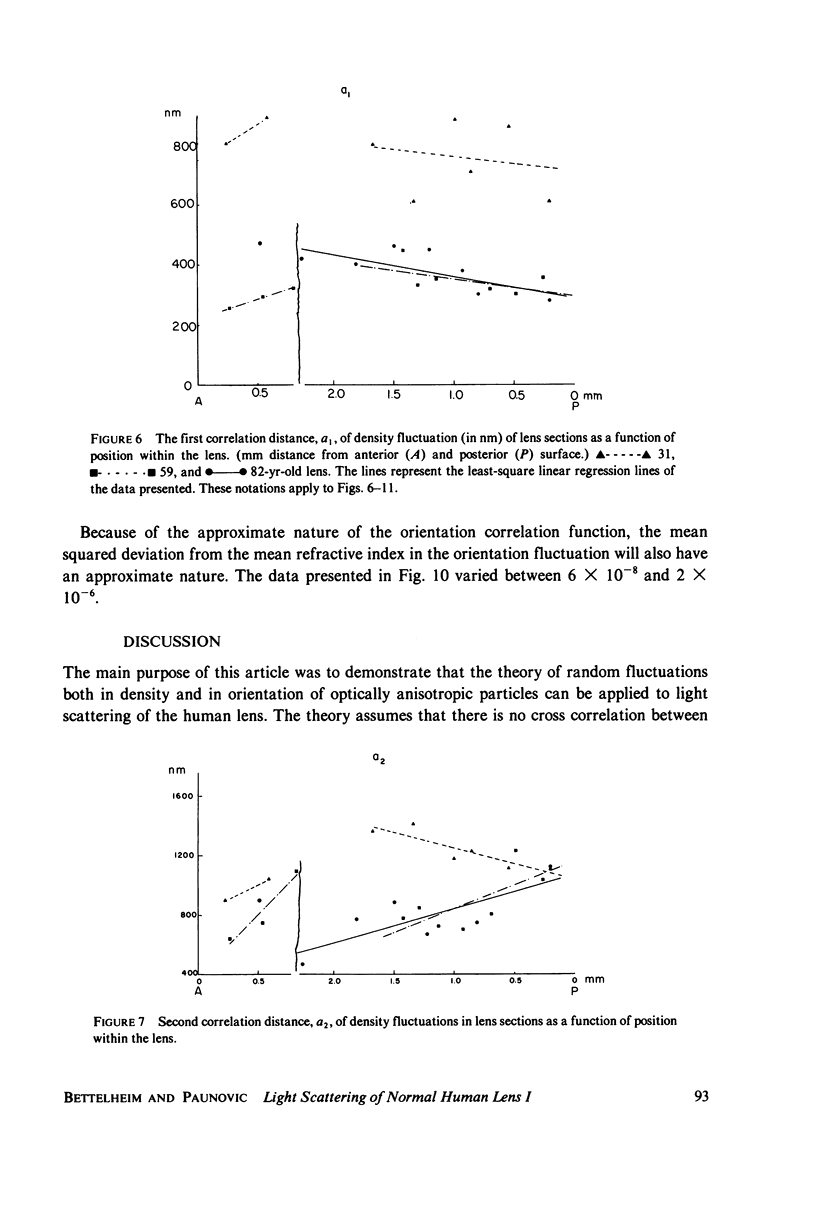
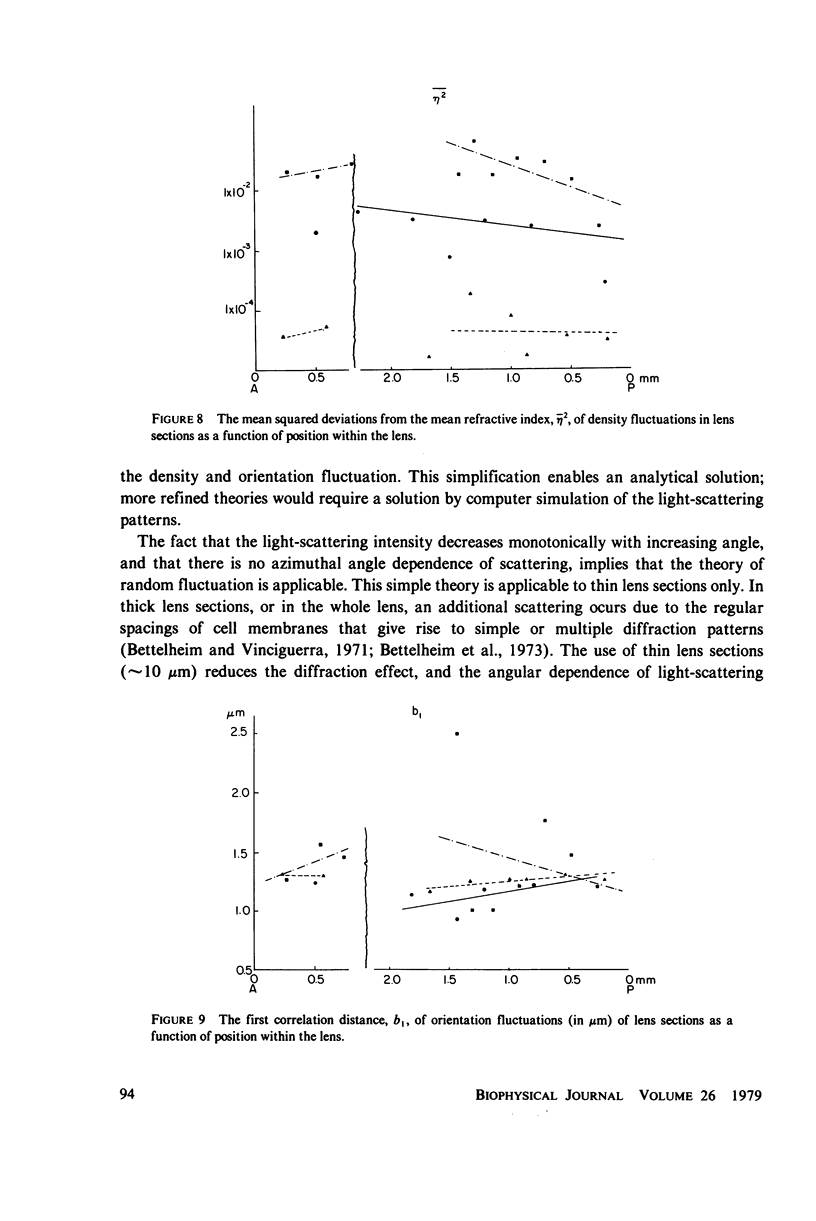
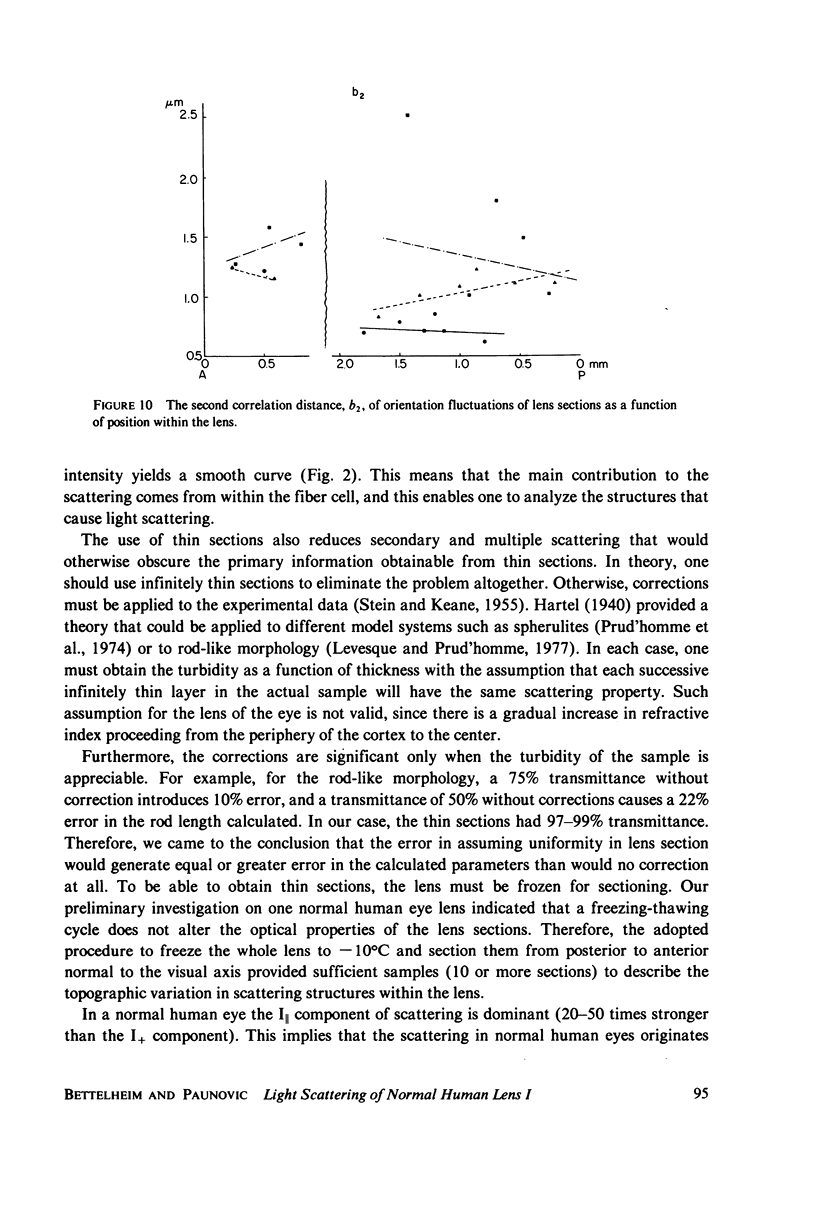

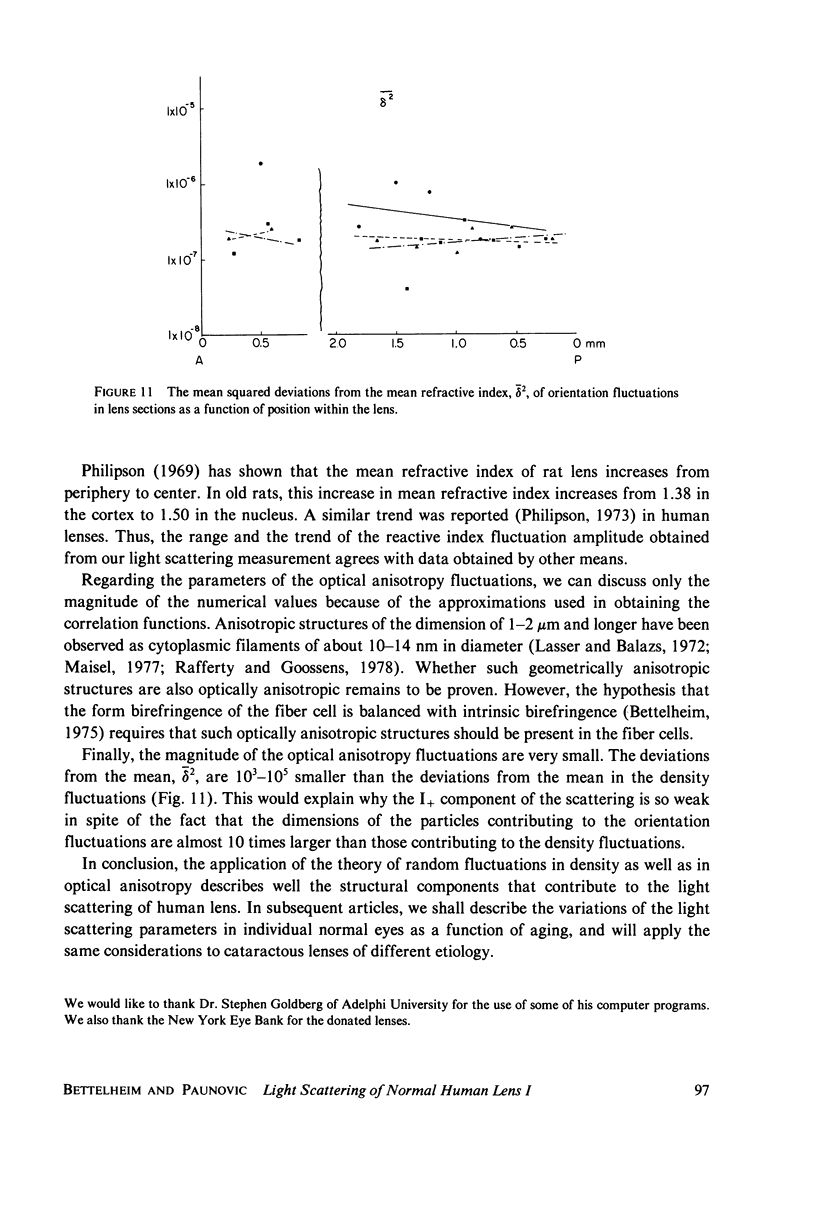


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettelheim F. A., Bettelheim A. A. Small-angle light scattering studies on xylose cataract formation in bovine lenses. Invest Ophthalmol Vis Sci. 1978 Sep;17(9):896–902. [PubMed] [Google Scholar]
- Bettelheim F. A. On the optical anisotropy of lens fiber cells. Exp Eye Res. 1975 Sep;21(3):231–234. doi: 10.1016/0014-4835(75)90093-7. [DOI] [PubMed] [Google Scholar]
- Bettelheim F. A., Vinciguerra M. J., Kaplan D. Dynamic laser diffraction of bovine lenses. Exp Eye Res. 1973 Feb;15(2):149–155. doi: 10.1016/0014-4835(73)90113-9. [DOI] [PubMed] [Google Scholar]
- Bettelheim F. A., Vinciguerra M. J. Laser-diffraction patterns of highly ordered superstructures in the lenses of bovine eyes. Ann N Y Acad Sci. 1971 May 28;172(11):429–439. doi: 10.1111/j.1749-6632.1971.tb34944.x. [DOI] [PubMed] [Google Scholar]
- Jedziniak J. A., Kinoshita J. H., Yates E. M., Hocker L. O., Benedek G. B. On the presence and mechanism of formation of heavy molecular weight aggregates in human normal and cataractous lenses. Exp Eye Res. 1973 Feb;15(2):185–192. doi: 10.1016/0014-4835(73)90118-8. [DOI] [PubMed] [Google Scholar]
- Kramps H. A., Stols A. L., Hoenders H. J. On the quaternary structure of high-molecular-weight proteins from the bovine eye lens. Eur J Biochem. 1975 Jan 15;50(3):503–509. doi: 10.1111/j.1432-1033.1975.tb09889.x. [DOI] [PubMed] [Google Scholar]
- Lasser A., Balazs E. A. Biochemical and fine structure studies on the water-insoluble components of the calf lens. Exp Eye Res. 1972 May;13(3):292–308. doi: 10.1016/0014-4835(72)90111-x. [DOI] [PubMed] [Google Scholar]
- Maisel H., Perry M. M. Electron microscope observations on some structural proteins of the chick lens. Exp Eye Res. 1972 Jul;14(1):7–12. doi: 10.1016/0014-4835(72)90136-4. [DOI] [PubMed] [Google Scholar]
- Maisel H. The effect of urea on the lens intracellular matrix and soluble lens protein. Exp Eye Res. 1977 Dec;25(6):595–601. doi: 10.1016/0014-4835(77)90138-5. [DOI] [PubMed] [Google Scholar]
- Perry M. M. A method to demonstrate the fine structural components of lens fibre cells. Exp Eye Res. 1976 Feb;22(2):125–128. doi: 10.1016/0014-4835(76)90039-7. [DOI] [PubMed] [Google Scholar]
- Philipson B. Changes in the lens related to the reduction of transparency. Exp Eye Res. 1973 Jun;16(1):29–39. doi: 10.1016/0014-4835(73)90234-0. [DOI] [PubMed] [Google Scholar]
- Philipson B. Distribution of protein within the normal rat lens. Invest Ophthalmol. 1969 Jun;8(3):258–270. [PubMed] [Google Scholar]
- Rafferty N. S., Esson E. A. An electron-microscope study of adult mouse lens: some ultrastructural specializations. J Ultrastruct Res. 1974 Feb;46(2):239–253. doi: 10.1016/s0022-5320(74)80059-6. [DOI] [PubMed] [Google Scholar]
- Rafferty N. S., Goossens W. Cytoplasmic filaments in the crystalline lens of various species: functional correlations. Exp Eye Res. 1978 Feb;26(2):177–190. doi: 10.1016/0014-4835(78)90115-x. [DOI] [PubMed] [Google Scholar]
- Spector A., Freund T., Li L. K., Augusteyn R. C. Age-dependent changes in the structure of alpha crystallin. Invest Ophthalmol. 1971 Sep;10(9):677–686. [PubMed] [Google Scholar]
- Spector A., Li S., Sigelman J. Age-dependent changes in the molecular size of human lens proteins and their relationship to light scatter. Invest Ophthalmol. 1974 Oct;13(10):795–798. [PubMed] [Google Scholar]
- THAEMERT J. C. Intercellular bridges as protoplasmic anastomoses between smooth muscle cells. J Biophys Biochem Cytol. 1959 Aug;6(1):67–70. doi: 10.1083/jcb.6.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROKEL S. The physical basis for transparency of the crystalline lens. Invest Ophthalmol. 1962 Aug;1:493–501. [PubMed] [Google Scholar]


