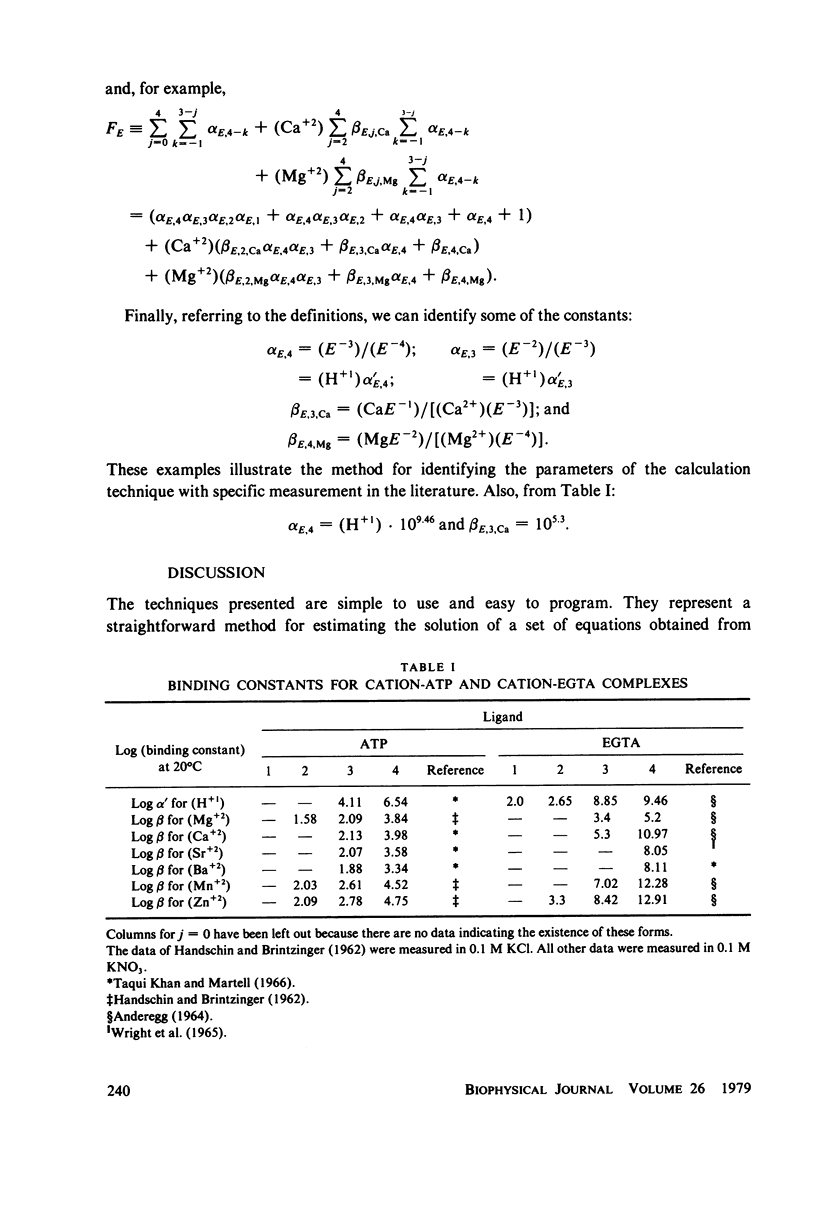
Abstract
The method described permits the computation of the concentrations of free ions and ion-ligand complexes in a solution containing arbitrary numbers of divalent cations and ligands. It is required that the pH be known, along with appropriate sets of ligand-hydrogen and ligand-divalent cation concentration binding constants. It is assumed that these sets of constants are chosen to be consistent with the ionic strength of the complete solution which contains the divalent cations and ligands. The technique is an iterative one which provides upper and lower bounds for the values of the unknowns. The method does not require initial guesses at the values of the unknowns, and it gives correct answers even when the concentrations involved are many orders of magnitude apart. The present formulation of the problem is restricted to the case where only one cation can bind to a given ligand at any one time. The method is applicable to large molecules with multiple "sub-ligands" provided these sub-ligands are independent in their function as ion-binding sites. These sub-ligands need not all have the same properties. It is also shown that a simple modification of the method permits the determination of the subset of total ion concentrations that are required in order to produce a specified subset of free ion concentrations. The modifications required to include monovalent cation binding are presented in outline form.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botts J., Chashin A., Schmidt L. Computation of metal binding in bi-metal--bi-chelate systems. Biochemistry. 1966 Apr;5(4):1360–1364. doi: 10.1021/bi00868a032. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M., Repke D. I., Upshaw J. E., Polascik M. A. Characterization of dog cardiac microsomes. Use of zonal centrifugation to fractionate fragmented sarcoplasmic reticulum, (Na+ + K+)--activated ATPase and mitochondrial fragments. Biochim Biophys Acta. 1970 Jun 30;205(3):473–490. doi: 10.1016/0005-2728(70)90113-1. [DOI] [PubMed] [Google Scholar]
- Khan M. M., Martell A. E. Thermodynamic quantities associated with the interaction of adenosine triphosphate with metal ions. J Am Chem Soc. 1966 Feb 20;88(4):668–671. doi: 10.1021/ja00956a008. [DOI] [PubMed] [Google Scholar]
- Perrin D. D. Multiple equilibria in assemblages of metal ions and complexing species: a model for biological systems. Nature. 1965 Apr 10;206(980):170–171. doi: 10.1038/206170a0. [DOI] [PubMed] [Google Scholar]


