Abstract
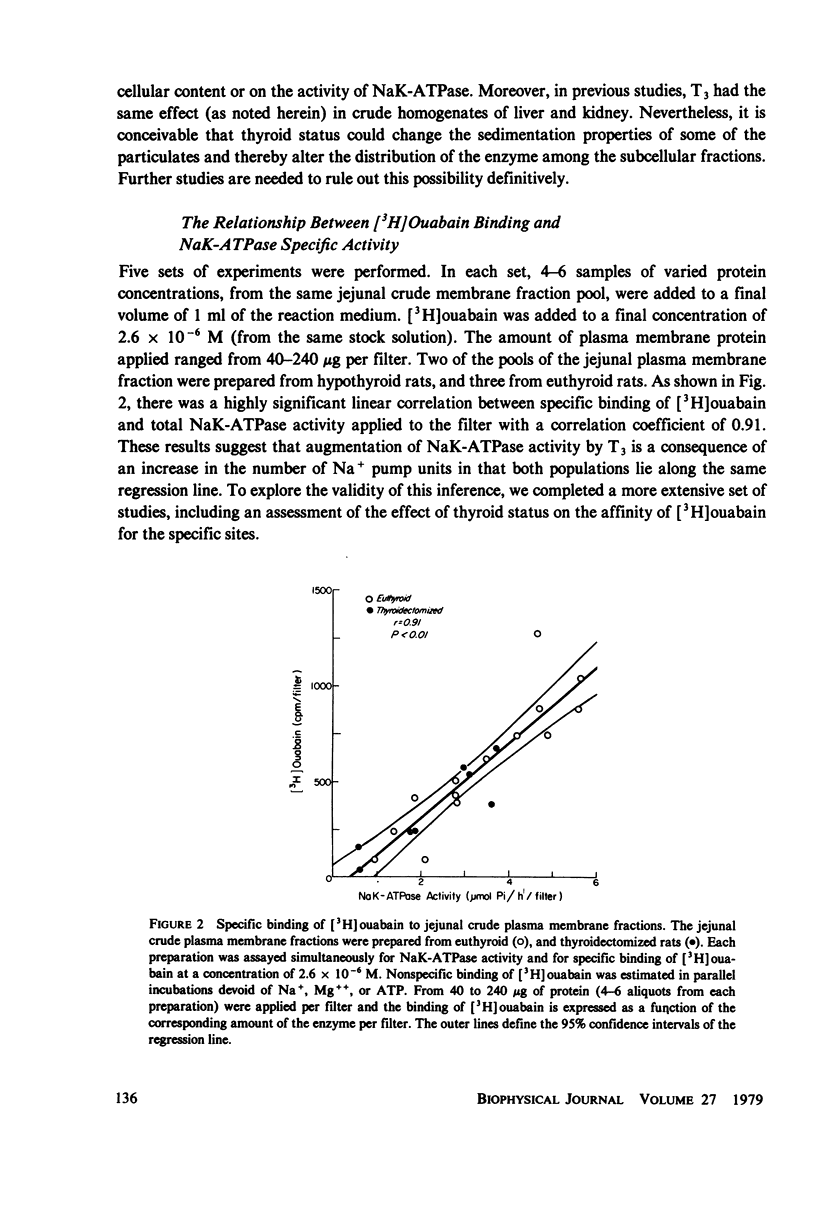
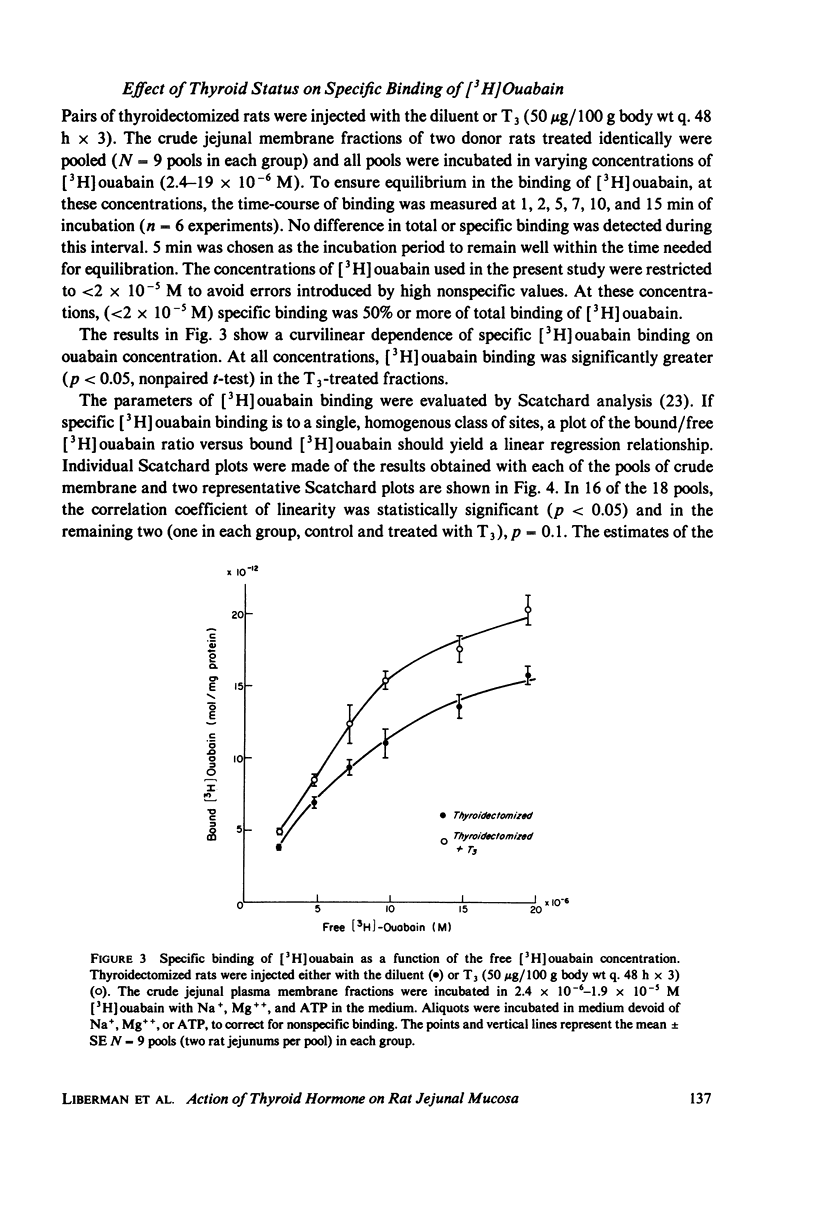
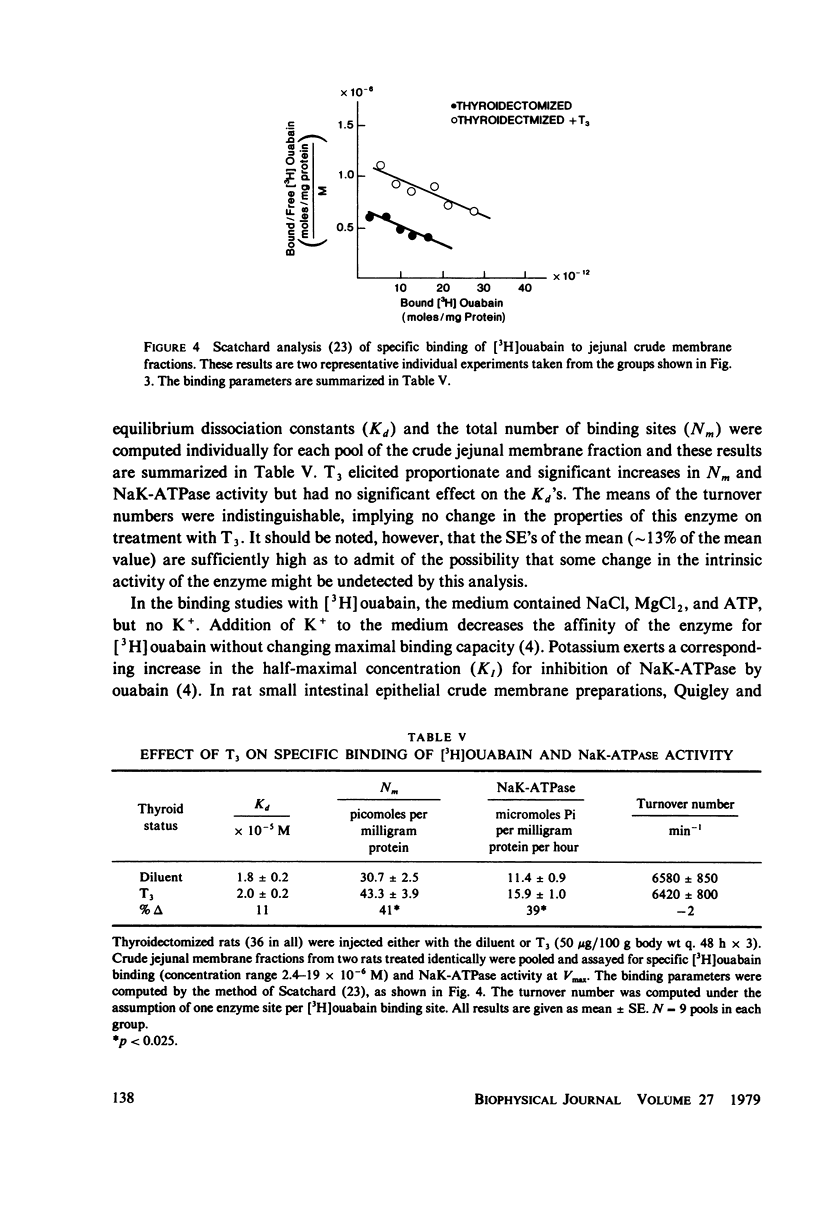
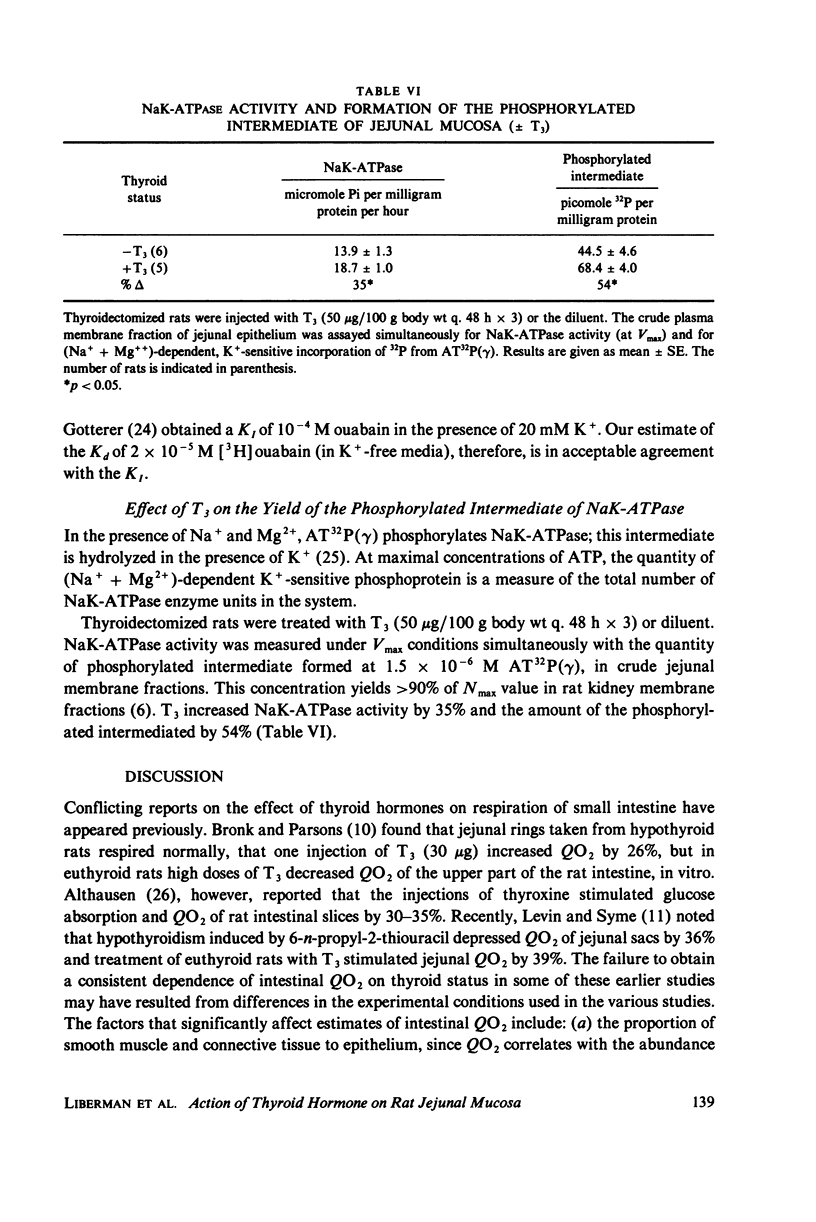
Administration of three successive doses of triiodothyronine (T3) (50 micrograms/100 g body wt), given on alternate days to thyroidectomized and euthyroid rats, stimulated oxygen consumption (QO2) and Na+ transport-dependent respiration (QO2 [5]) in the stripped jejunal mucosa, a preparation that consisted mostly of epithelial cells. The increase in QO2(t) accounted for 57% of the increment in QO2 in the transition from the hypothyroid to the euthyroid state and for 29% of the increment in the transition from the euthyroid to the hyperthyroid state. Administration of T3 to hypothyroid rats also increased the yield of epithelial cells. Injection of T3 into thyroidectomized and euthyroid rats increased the specific activity (at Vmax) of the (Na+ + K+)-dependent adenosine triphosphatase (NaK-ATPase) in jejunal crude membrane preparations. No significant change was recorded in the activity of Mg-ATPase in the same preparation. The ratio of QO2/NaK-ATPase and QO2(t)/NaK-ATPase in the various thyroid states remained constant, indicating proportionate increased in the respiratory and enzymatic indices. The effect of administration of T3 to thyroidectomized rats on the number of NaK-ATPase units (recovered in the crude membrane preparation) was estimated by: (a) Na+ + Mg++ + ATP-dependent binding of [3H]-ouabain to crude membrane fractions, and (b) the amount of the phosphorylated intermediate formed in the NaK-ATPase reaction from AT32P(gamma). Estimates were obtained of the maximal number of [3H]ouabain binding sites (Nm) and dissociation constants (Kd). Nm for [3H]ouabain and Nak-ATPase specific activity increased to about the same extent after T3 administration to thyroidectomized rats, with no change in the apparent Kd values. The amount of phosphorylated intermediate formed in jejunal crude membrane preparations also increased significantly. Thus, thyroid hormone administration may increase the number of active Na+pump sites in the plasma membrane. The apparent increase in the number of Na+ pump sites also correlated with the hormone dependent increases in QO2 and QO2(t).
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. C., Schwartz A. A possible biochemical explanation for the insensitivity of the rat to cardiac glycosides. J Pharmacol Exp Ther. 1969 Jul;168(1):42–46. [PubMed] [Google Scholar]
- Asano Y., Liberman U. A., Edelman I. S. Thyroid thermogenesis. Relationships between Na+-dependent respiration and Na+ + K+-adenosine triphosphatase activity in rat skeletal muscle. J Clin Invest. 1976 Feb;57(2):368–379. doi: 10.1172/JCI108288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER S. B. Effect of triiodothyronine on oxygen consumption of tissues not responsive to thyroxine. Proc Soc Exp Biol Med. 1955 Oct;90(1):109–111. doi: 10.3181/00379727-90-21954. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader H., Post R. L., Bond G. H. Comparison of sources of a phosphorylated intermediate in transport ATPase. Biochim Biophys Acta. 1968 Jan 3;150(1):41–46. doi: 10.1016/0005-2736(68)90006-0. [DOI] [PubMed] [Google Scholar]
- Birge S. J., Jr, Gilbert H. R., Avioli L. V. Intestinal calcium transport: the role of sodium. Science. 1972 Apr 14;176(4031):168–170. doi: 10.1126/science.176.4031.168. [DOI] [PubMed] [Google Scholar]
- Bronk J. R., Parsons D. S. Influence of the thyroid gland on the accumulation of sugars in rat intestinal mucosa during absorption. J Physiol. 1965 Jul;179(2):323–332. doi: 10.1113/jphysiol.1965.sp007665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere R. M. The influence of thyroid and testicular hormones on the epithelium of crypts of Lieberkühn in the rat's intestine. Anat Rec. 1966 Dec;156(4):423–431. doi: 10.1002/ar.1091560406. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi F., Dietz T., Edelman I. S. Thyroid thermogenesis: minimal contribution of energy requirement for protein synthesis. Mol Cell Endocrinol. 1976 Jun-Jul;5(1-2):19–22. doi: 10.1016/0303-7207(76)90066-6. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi F., Edelman I. S. Effects of thyroid status on electrolyte distribution in rat tissues. Am J Physiol. 1973 Nov;225(5):1172–1177. doi: 10.1152/ajplegacy.1973.225.5.1172. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi F., Edelman I. S. The mechanism of the calorigenic action of thyroid hormone. Stimulation of Na plus + K plus-activated adenosinetriphosphatase activity. J Gen Physiol. 1971 Jun;57(6):710–722. doi: 10.1085/jgp.57.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail-Beigi F., Edelman I. S. Time-course of the effects of thyroid hormone on respiration and Na+ + K+-ATPase activity in rat liver. Proc Soc Exp Biol Med. 1974 Sep;146(4):983–988. doi: 10.3181/00379727-146-38232. [DOI] [PubMed] [Google Scholar]
- Izmail-Beigi F., Edelman I. S. Mechanism of thyroid calorigenesis: role of active sodium transport. Proc Natl Acad Sci U S A. 1970 Oct;67(2):1071–1078. doi: 10.1073/pnas.67.2.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D. T., Sippel A. E., Feigelson P. Effect of thyroid hormones on the level of the hepatic mRNA for alpha2u globulin. Biochemistry. 1976 Mar 9;15(5):1031–1036. doi: 10.1021/bi00650a013. [DOI] [PubMed] [Google Scholar]
- LEVIN R. J., SMYTH D. H. THE EFFECT OF THE THYROID GLAND ON INTESTINAL ABSORPTION OF HEXOSES. J Physiol. 1963 Dec;169:755–769. doi: 10.1113/jphysiol.1963.sp007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin F. J., Syme G. Thyroid control of small intestinal oxygen consumption and the influence of sodium ions, oxyhen tension, glucose and anaesthesia. J Physiol. 1975 Feb;245(1):271–287. doi: 10.1113/jphysiol.1975.sp010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. J. The effects of hormones on the absorptive, metabolic and digestive functions of the small intestine. J Endocrinol. 1969 Oct;45(2):315–348. doi: 10.1677/joe.0.0450315. [DOI] [PubMed] [Google Scholar]
- Lin M. H., Akera T. Increased (Na+,K+)-ATPase concentrations in various tissues of rats caused by thyroid hormone treatment. J Biol Chem. 1978 Feb 10;253(3):723–726. [PubMed] [Google Scholar]
- Lo C. S., Edelman I. S. Effect of triiodothyronine on the synthesis and degradation of renal cortical (Na+ + k+)-adenosine triphosphatase. J Biol Chem. 1976 Dec 25;251(24):7834–7840. [PubMed] [Google Scholar]
- Lo S. C., August T. R., Liberman U. A., Edelman I. S. Dependence of renal (Na+ + k+)-adenosine triphosphatase activity on thyroid status. J Biol Chem. 1976 Dec 25;251(24):7826–7833. [PubMed] [Google Scholar]
- MURPHY B. E., PATTEE C. J. DETERMINATION OF THYROXINE UTILIZING THE PROPERTY OF PROTEIN-BINDING. J Clin Endocrinol Metab. 1964 Feb;24:187–196. doi: 10.1210/jcem-24-2-187. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck B. G. Effects of sugar and amino acid transport on transepithelial fluxes of sodium and chloride of short circuited rat jejunum. J Physiol. 1972 Jun;223(3):699–717. doi: 10.1113/jphysiol.1972.sp009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- POST R. L., SEN A. K., ROSENTHAL A. S. A PHOSPHORYLATED INTERMEDIATE IN ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT ACROSS KIDNEY MEMBRANES. J Biol Chem. 1965 Mar;240:1437–1445. [PubMed] [Google Scholar]
- Philipson K. D., Edelman I. S. Characteristics of thyroid-stimulated Na+-K+-ATPase of rat heart. Am J Physiol. 1977 May;232(5):C202–C206. doi: 10.1152/ajpcell.1977.232.5.C202. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Edelman I. S. Thyroid hormone control of Na+-K+-ATPase and K+-dependent phosphatase in rat heart. Am J Physiol. 1977 May;232(5):C196–C201. doi: 10.1152/ajpcell.1977.232.5.C196. [DOI] [PubMed] [Google Scholar]
- Pietra P., Cappelli V. Evaluation of O2 availability during glucose transport in everted sacs of rat small intestine. Experientia. 1970 May 15;26(5):514–515. doi: 10.1007/BF01898479. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Gotterer G. S. A comparison of the (Na + -K + )-ATPase activities found in isolated brush border and plasma membrane of the rat intestinal mucosa. Biochim Biophys Acta. 1972 Jan 17;255(1):107–113. doi: 10.1016/0005-2736(72)90012-0. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Gotterer G. S. Distribution of (Na+-K+)-stimulated ATPase activity in rat intestinal mucosa. Biochim Biophys Acta. 1969 Apr;173(3):456–468. doi: 10.1016/0005-2736(69)90010-8. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Gotterer G. S. Properties of a high specific activity, (Na+-K+)-stimulated ATPase from rat intestinal mucosa. Biochim Biophys Acta. 1969 Apr;173(3):469–476. doi: 10.1016/0005-2736(69)90011-x. [DOI] [PubMed] [Google Scholar]
- Rahimifar M., Ismail-Beigi Lack of thyroid hormone effect on activation energy of NaK-ATPase. Mol Cell Endocrinol. 1977 Feb;6(4-5):327–331. doi: 10.1016/0303-7207(77)90107-1. [DOI] [PubMed] [Google Scholar]
- SESHADRI B. ACTION OF THYROXINE ON THE INTESTINAL TISSUE OF RAT. Indian J Exp Biol. 1965 Apr;3:97–99. [PubMed] [Google Scholar]
- Tobin T., Henderson R., Sen A. K. Species and tissue differences in the rate of dissociation of ouabain from (Na+ + K+)-ATPase. Biochim Biophys Acta. 1972 Aug 9;274(2):551–555. doi: 10.1016/0005-2736(72)90201-5. [DOI] [PubMed] [Google Scholar]




