Abstract
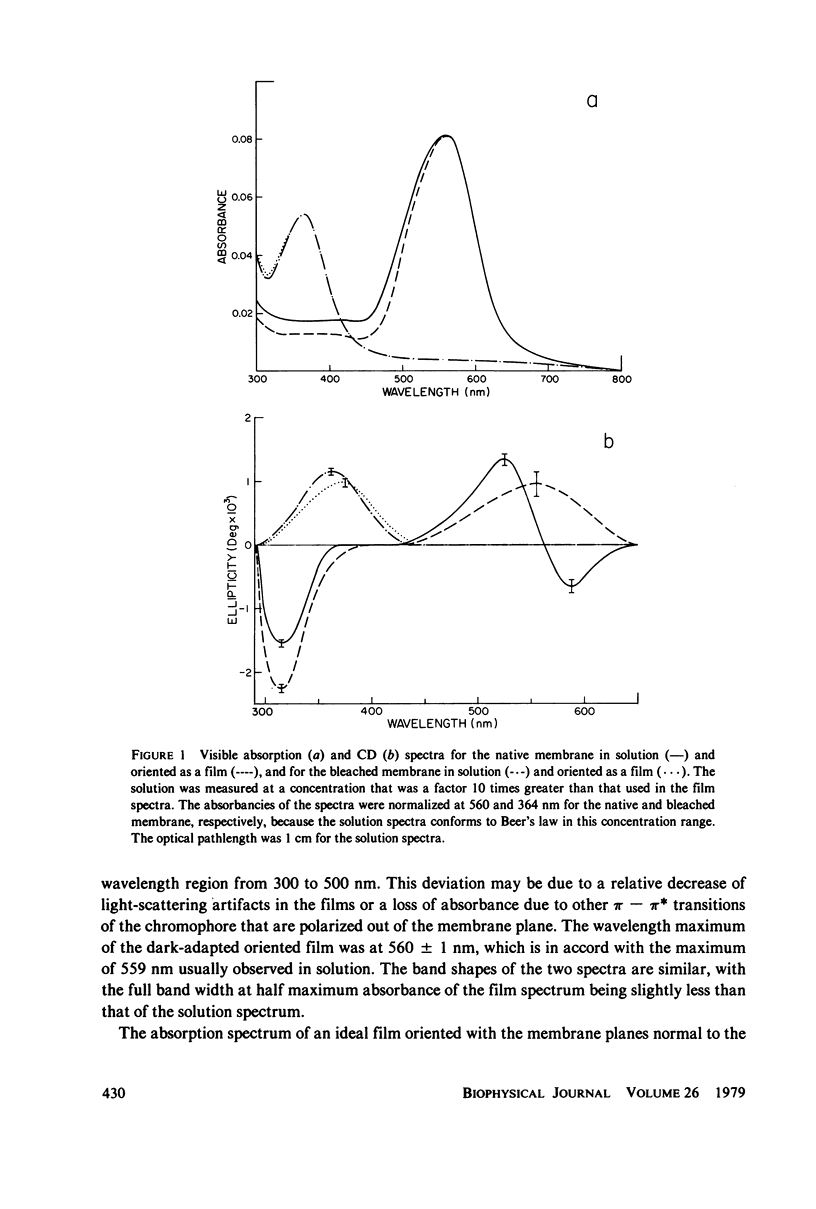
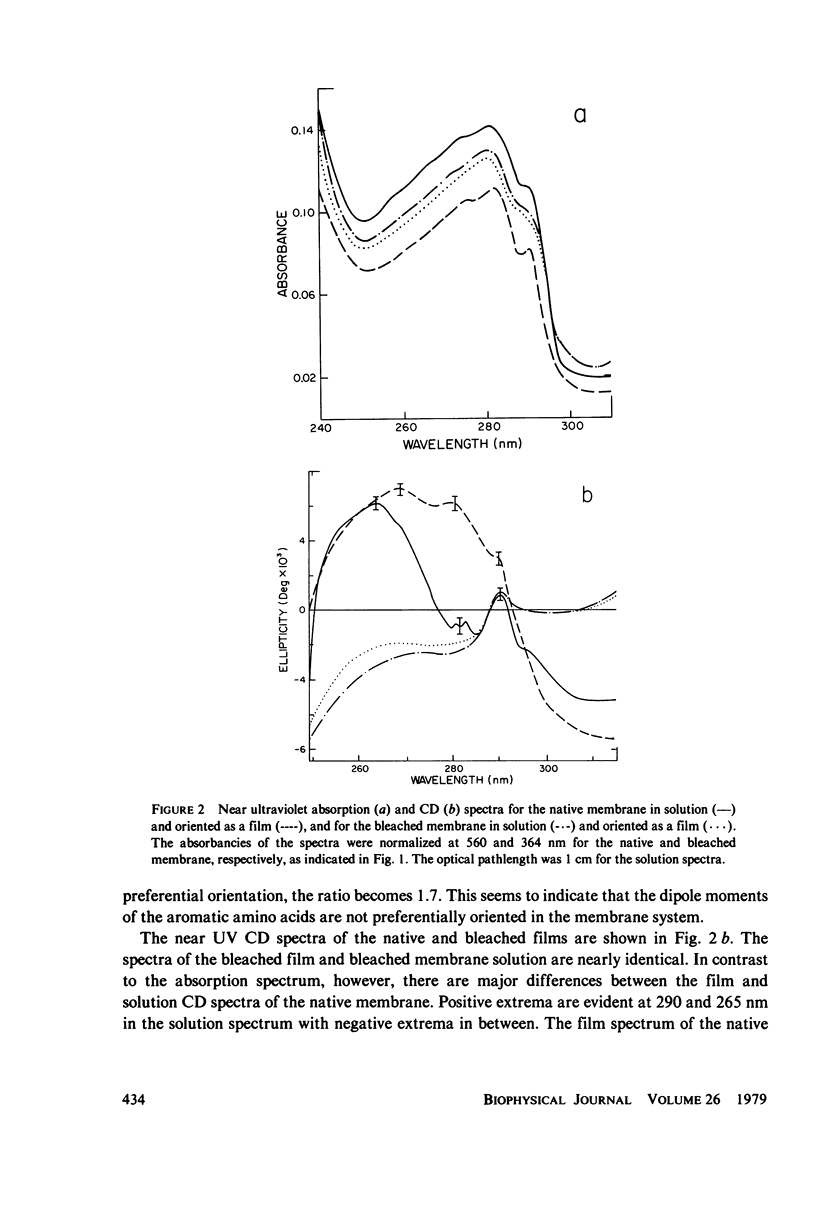
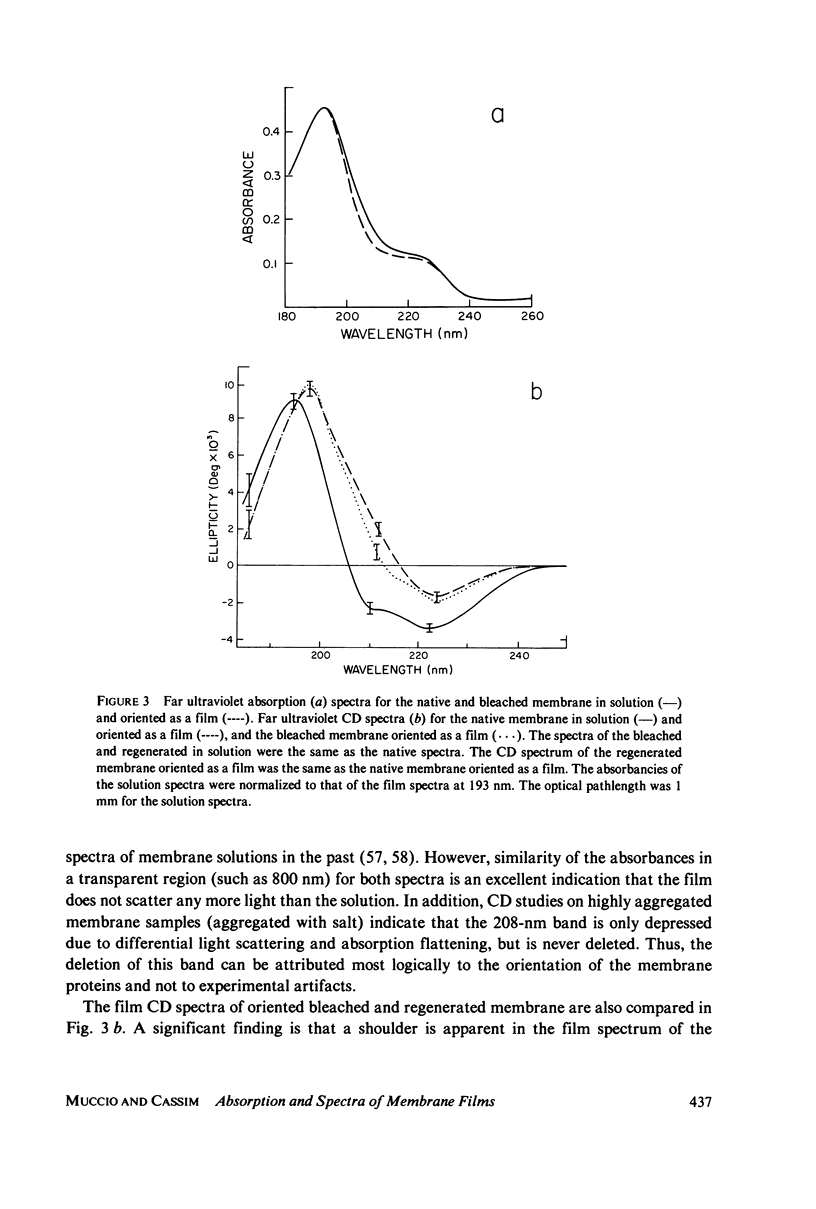
The absorption and circular dichroic (CD) spectra of purple membrane films in which the plane of the membranes is oriented perpendicular to the incident beam are compared with the solution spectra. This enables one to relate structural features of the purple membrane to a coordinate system as defined by a normal to the membrane plane and two mutually perpendicular in-plane axes. The film and solution absorption spectra were similar except for a relative depression in the 200 - 225-nm region of the film spectrum. However, the CD spectra showed significant differences in the visible region, where the biphasic band in the solution spectrum was replaced by a single positive band at 555 nm in the film spectrum and in the far ultraviolet region, where the 208-nm band was deleted from the film spectra of the native and regenerated membranes. Moreover, a small shoulder occurred at 208 nm in the film spectrum of the bleached membrane. The near ultraviolet spectra also showed differences, whereas the 317-nm band remained essentially the same for both spectra. Based on excitonic interpretations of the visible and far ultraviolet spectra the following conclusions were reached: (a) a relatively strong in-plane monomeric interaction occurs between te retinyl chromophore and apoprotein; (b) the helical axes of the native and regenerated membrane proteins are oriented primarily normal to the membrane plane; and (c) the helical axes of the bleached membrane proteins are tilted more in-plane than the axes of the native or regenerated membrane. Additional conclusions were that an interaction occurs between an in-plane magnetic dipole moment of the retinyl chromophore and probably an in-plane electric dipole moment of a nearby aromatic amino acid(s), and that although the membrane is anisotropic with respect to coupling between electric and magnetic moments of the aromatic amino acids, the transition dipole moments of the aromatic amino acids are not preferentially oriented in either direction.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of bleaching and regeneration on the purple membrane structure of Halobaterium halobium. Biophys J. 1977 Sep;19(3):285–297. doi: 10.1016/s0006-3495(77)85588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of light adaptation on the purple membrane structure of Halobacterium halobium. Biophys J. 1976 Oct;16(10):1183–1200. doi: 10.1016/S0006-3495(76)85767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. Evidence for chromophore-chromophore (exciton) interaction in the purple membrane of Halobacterium halobium. Biochem Biophys Res Commun. 1976 Mar 8;69(1):1–6. doi: 10.1016/s0006-291x(76)80263-x. [DOI] [PubMed] [Google Scholar]
- Becher B., Tokunaga F., Ebrey T. G. Ultraviolet and visible absorption spectra of the purple membrane protein and the photocycle intermediates. Biochemistry. 1978 Jun 13;17(12):2293–2300. doi: 10.1021/bi00605a006. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Stubbs L., Lanyi J. K. Illumination-dependent changes in the intrinsic fluorescence of bacteriorhodopsin. Biochemistry. 1978 Mar 21;17(6):1037–1041. doi: 10.1021/bi00599a015. [DOI] [PubMed] [Google Scholar]
- Brahms J., Pilet J., Damany H., Chandrasekharan V. Application of a new modulation method for linear dichroism studies of oriented biopolymers in the vacuum ultraviolet. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1130–1137. doi: 10.1073/pnas.60.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T. G., Becher B., Mao B., Kilbride P., Honig B. Exciton interactions and chromophore orientation in the purple membrane. J Mol Biol. 1977 May 25;112(3):377–397. doi: 10.1016/s0022-2836(77)80188-5. [DOI] [PubMed] [Google Scholar]
- Fasman G. D., Hoving H., Timasheff S. N. Circular dichroism of polypeptide and protein conformations. Film studies. Biochemistry. 1970 Aug 18;9(17):3316–3324. doi: 10.1021/bi00819a005. [DOI] [PubMed] [Google Scholar]
- Gordon D. J. Mie scattering by optically active particles. Biochemistry. 1972 Feb 1;11(3):413–420. doi: 10.1021/bi00753a018. [DOI] [PubMed] [Google Scholar]
- Gratzer W. B., Holzwarth G. M., Doty P. POLARIZATION OF THE ULTRAVIOLET ABSORPTION BANDS IN alpha-HELICAL POLYPEPTIDES. Proc Natl Acad Sci U S A. 1961 Nov;47(11):1785–1791. doi: 10.1073/pnas.47.11.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZWARTH G., DOTY P. THE ULTRAVIOLET CIRCULAR DICHROISM OF POLYPEPTIDES. J Am Chem Soc. 1965 Jan 20;87:218–228. doi: 10.1021/ja01080a015. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Bauer P. J., Dencher N. A. A natural CD label to probe the structure of the purple membrane from Halobacterium halobium by means of exciton coupling effects. Biochem Biophys Res Commun. 1975 Dec 1;67(3):897–903. doi: 10.1016/0006-291x(75)90761-5. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. M., Urry D. W., Stoeckenius W. Circular dichroism of biological membranes: purple membrane of Halobacterium halobium. Biochem Biophys Res Commun. 1977 Apr 11;75(3):725–731. doi: 10.1016/0006-291x(77)91532-7. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt W., Fitts D. D., Kirkwood J. G. CRITIQUE OF THE THEORY OF OPTICAL ACTIVITY OF HELICAL POLYMERS. Proc Natl Acad Sci U S A. 1957 Aug 15;43(8):723–730. doi: 10.1073/pnas.43.8.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Meentzen M., Schuhmann L. Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans-retinal as its chromophores. Eur J Biochem. 1973 Dec 17;40(2):453–463. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Schuhmann L. Reconstitution of bacteriorhodopsin. FEBS Lett. 1974 Aug 30;44(3):262–265. doi: 10.1016/0014-5793(74)81153-1. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Papadopoulos G. K., Hsiao T. L., Cassim J. Y. Determination of the retinal/protein molar ratios for the purple membranes of Halobacterium halobium and Halobacterium cutirubrum. Biochem Biophys Res Commun. 1978 Mar 15;81(1):127–132. doi: 10.1016/0006-291x(78)91639-x. [DOI] [PubMed] [Google Scholar]
- Pettei M. J., Yudd A. P., Nakanishi K., Henselman R., Stoeckenius W. Identification of retinal isomers isolated from bacteriorhodopsin. Biochemistry. 1977 May 3;16(9):1955–1959. doi: 10.1021/bi00628a031. [DOI] [PubMed] [Google Scholar]
- Schneider A. S. Analysis of optical activity spectra of turbid biological suspensions. Methods Enzymol. 1973;27:751–767. doi: 10.1016/s0076-6879(73)27032-5. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H. Light energy conversion in Halobacterium halobium. J Supramol Struct. 1974;2(5-6):769–774. doi: 10.1002/jss.400020519. [DOI] [PubMed] [Google Scholar]
- Sumper M., Reitmeier H., Oesterhelt D. Biosynthesis of the purple membrane of halobacteria. Angew Chem Int Ed Engl. 1976 Apr;15(4):187–194. doi: 10.1002/anie.197601871. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Woody R. W. Improved calculation of the n-pi rotational strength in polypeptides. J Chem Phys. 1968 Dec 1;49(11):4797–4806. doi: 10.1063/1.1669962. [DOI] [PubMed] [Google Scholar]


