Abstract
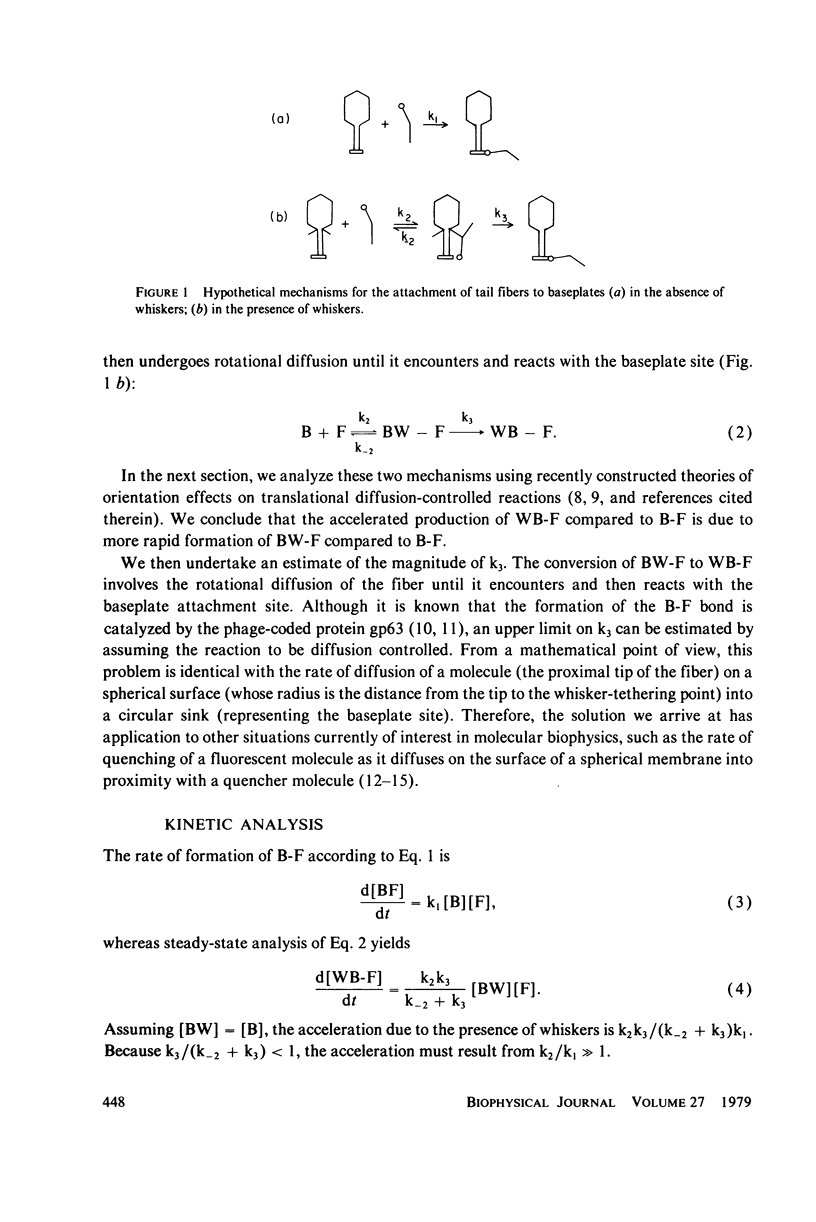
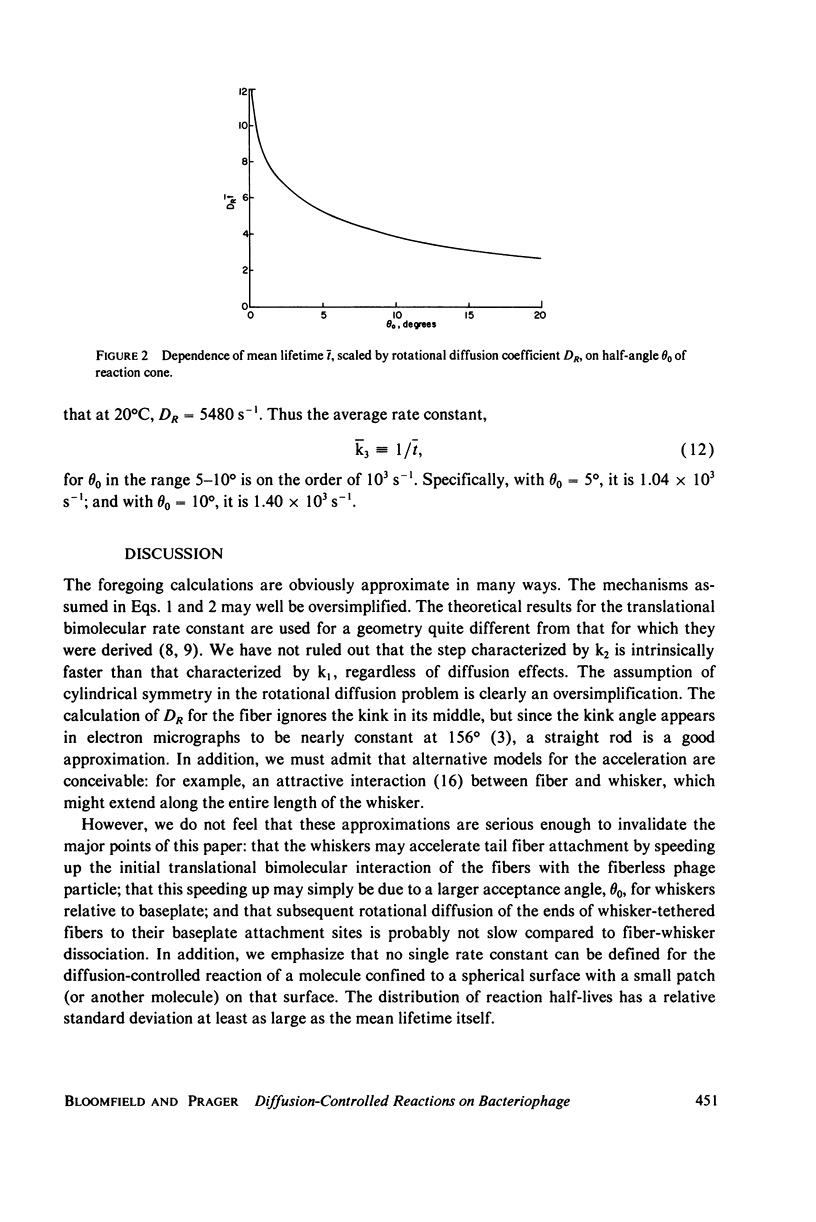
We have explored the kinetic implications of a model that may account for the acceleration of tail fiber (F) attachment to baseplates (B) by whiskers (W) on bacteriophage T4. The model assumes that a W-F complex is formed initially, and that the tethered fiber then undergoes rotational diffusion until a B-F encounter takes place. In the absence of whiskers, B-F complexes must form unassisted. Formation of a W-F intermediate will accelerate F attachment to B if (a) the bimolecular rate constant for W-F complex formation is larger than that for direct B-F interaction and (b) subsequent rotational diffusion of the tip of F to B is not much slower than the dissociation of W-F. Condition a was investigated by applying a recent theory of orientational effects on translational diffusion-controlled reactions. This theory suggests that substantial rate enhancement is expected if the reaction half-angle theta 0 is larger for W-F than for B-F complex formation. Condition b was investigated by calculating the mean and the variance of the time required for the diffusion of a molecule (the proximal tip of the fiber) on a spherical surface (whose radius is the distance from the tip to the whisker tethering point) into a circular sink (the baseplate site). The mean time is on the order of the inverse rotational diffusion coefficient, DR, of the fiber, but is sensitive to theta 0. Both conditions are satisfied for plausible choices of parameters. The solution to the diffusion equation we have obtained should have application to other physical situations, such as the rate of quenching of a fluorophore as it diffuses on the surface of a spherical membrane into proximity with a quencher.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop R. J., Conley M. P., Wood W. B. Assembly and attachment of bacteriophage T4 tail fibers. J Supramol Struct. 1974;2(2-4):196–201. doi: 10.1002/jss.400020214. [DOI] [PubMed] [Google Scholar]
- Conley M. P., Wood W. B. Bacteriophage T4 whiskers: a rudimentary environment-sensing device. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3701–3705. doi: 10.1073/pnas.72.9.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey M. J., Wiberg J. S., Frankel F. R. Genetic control of whisker antigen of bacteriophage T4D. J Mol Biol. 1974 Apr 25;84(4):625–634. doi: 10.1016/0022-2836(74)90120-x. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- King J., Wood W. B. Assembly of bacteriophage T4 tail fibers: the sequence of gene product interaction. J Mol Biol. 1969 Feb 14;39(3):583–601. doi: 10.1016/0022-2836(69)90147-8. [DOI] [PubMed] [Google Scholar]
- Lardner T. J., Solomon N. The determination of local cell membrane diffusion coefficients. J Theor Biol. 1976 Aug 7;60(2):433–440. doi: 10.1016/0022-5193(76)90069-2. [DOI] [PubMed] [Google Scholar]
- Schurr J. M. The role of diffusion in bimolecular solution kinetics. Biophys J. 1970 Aug;10(8):700–716. doi: 10.1016/S0006-3495(70)86330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snopek T. J., Wood W. B., Conley M. P., Chen P., Cozzarelli N. R. Bacteriophage T4 RNA ligase is gene 63 product, the protein that promotes tail fiber attachment to the baseplate. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3355–3359. doi: 10.1073/pnas.74.8.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi E. Alternative pathways of tail fiber assembly in bacteriophage T4? J Mol Biol. 1971 Jul 28;59(2):319–327. doi: 10.1016/0022-2836(71)90053-2. [DOI] [PubMed] [Google Scholar]


