Abstract
Light diffraction patterns from isolated frog semitendinosus muscle fibers were examined. When transilluminated by laser light, the muscle striations produce a diffraction pattern consisting of a series of lines that are projected as points onto an optical detector by a lens system. Diffraction data may be sequentially stored every 18 ms for later processing by digital computer systems. First- and second-order diffraction line intensities were examined from intact, chemically skinned, and glycerinated single fibers. The diffraction line intensities demonstrated a strong length dependence upon passive stretch from reference length to 3.6 micrometer. The first-order intensity linearly increased an average of 15-fold over the range examined. The magnitude of the second order intensity was less than the first order and showed an exponential rise with increasing length. Both first- and second-order intensities decreased upon muscle activation. Data from chemically skinned and glycerinated single fibers were not significantly different from intact fibers, indicating that the membrane structure has little effect upon the diffraction phenomenon in muscle. Theoretical model systems are examined in an attempt to find the basis of these results. Neither an analysis based on a diffraction grating with variable spacing nor the unit cell model of Fujime provides an explanation for the observed length dependency of intensity. Though the origin of the intensity decrease upon stimulation is not known, we have suggested that it could result from lateral misalignment of myofibrils and can occur upon activation.
Full text
PDF






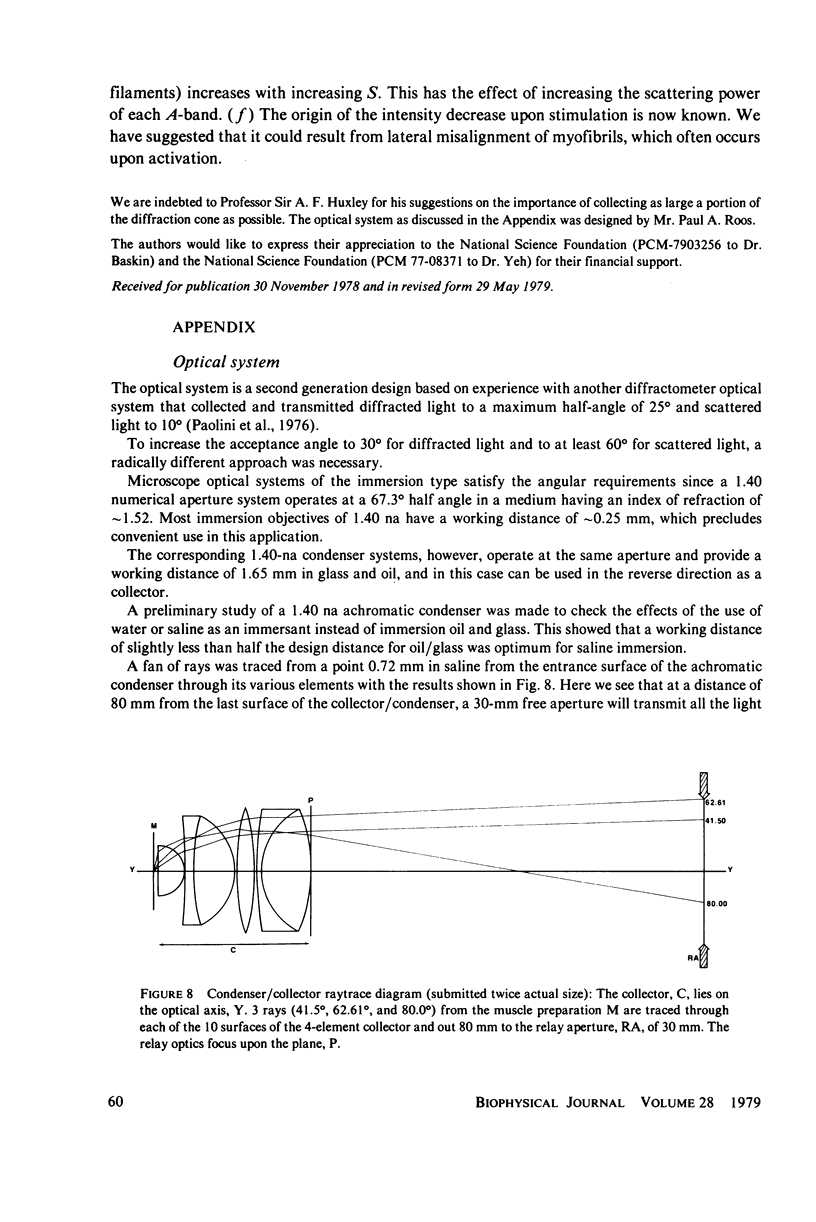
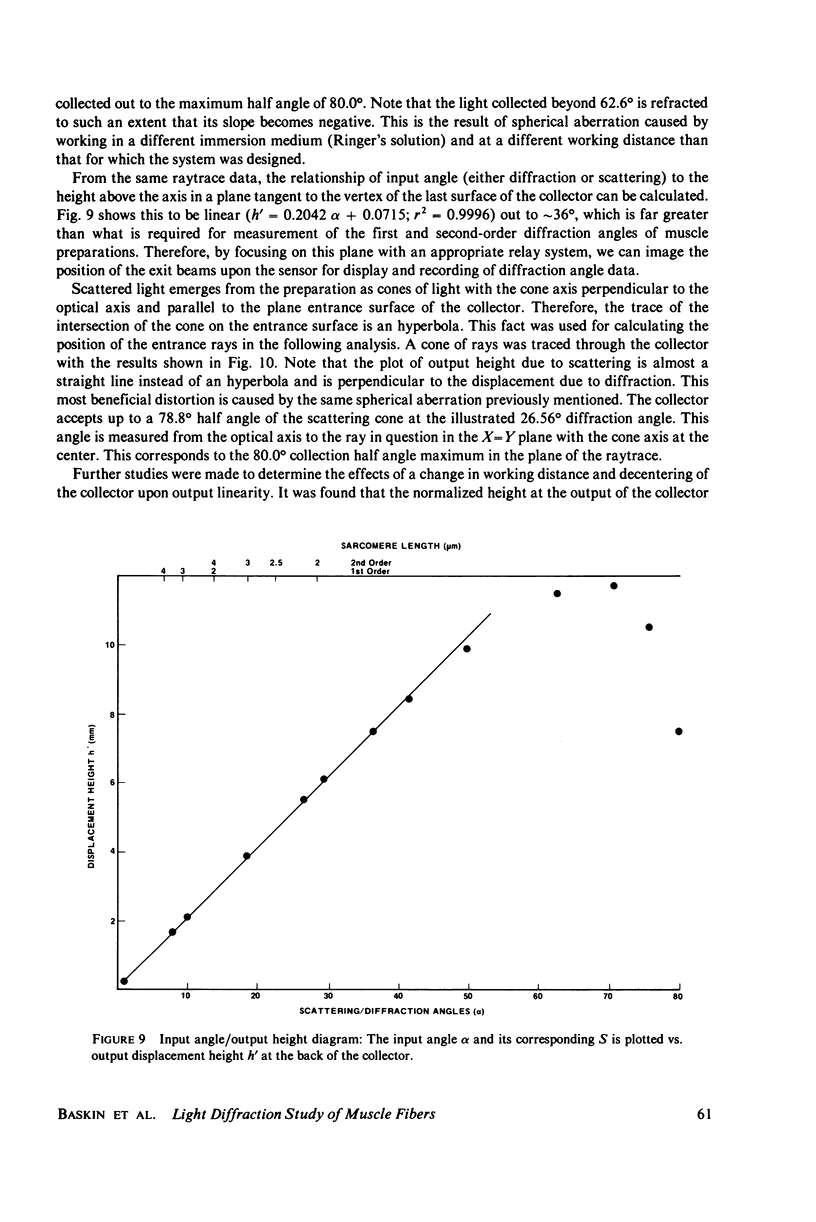
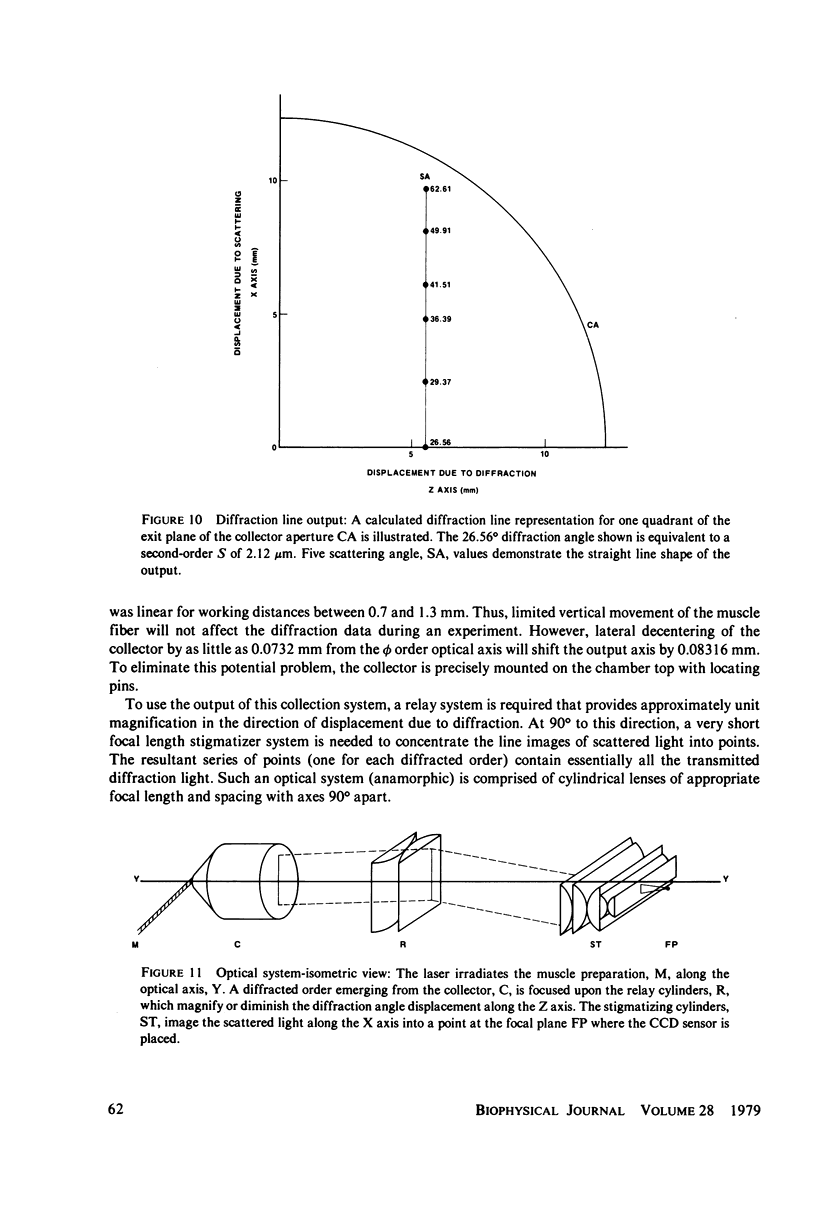


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner R. F., Carlson F. D. Structural dynamics of frog muscle during isometric contraction. J Gen Physiol. 1975 May;65(5):555–581. doi: 10.1085/jgp.65.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujime S. Optical diffraction study of muscle fibers. Biochim Biophys Acta. 1975 Jan 30;379(1):227–238. doi: 10.1016/0005-2795(75)90026-4. [DOI] [PubMed] [Google Scholar]
- HILL D. K. The effect of stimulation on the diffraction of light by striated muscle. J Physiol. 1953 Mar;119(4):501–512. doi: 10.1113/jphysiol.1953.sp004862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. The optical properties of resting striated muscle; the effect of rapid stretch on the scattering and diffraction of light. J Physiol. 1953 Mar;119(4):489–500. doi: 10.1113/jphysiol.1953.sp004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. X-ray analysis and the problem of muscle. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):59–62. doi: 10.1098/rspb.1953.0017. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J Mol Biol. 1975 Feb 15;92(1):113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- Hill L. A-band length, striation spacing and tension change on stretch of active muscle. J Physiol. 1977 Apr;266(3):677–685. doi: 10.1113/jphysiol.1977.sp011787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial x-ray diagram. J Mol Biol. 1968 Nov 14;37(3):507–520. doi: 10.1016/0022-2836(68)90118-6. [DOI] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Kuntz I. D. Optical diffraction studies of muscle fibers. Biophys J. 1973 Sep;13(9):857–876. doi: 10.1016/S0006-3495(73)86031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini P. J., Baskin R. J., Roos K. P., Cline J. W. Dual-channel diffractometer utilizing linear image sensor charge-coupled devices. Rev Sci Instrum. 1976 Jun;47(6):698–702. doi: 10.1063/1.1134711. [DOI] [PubMed] [Google Scholar]
- Paolini P. J., Roos K. P., Baskin R. J. Light diffraction studies of sarcomere dynamics in single skeletal muscle fibers. Biophys J. 1977 Nov;20(2):221–232. doi: 10.1016/S0006-3495(77)85545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini P. J., Sabbadini R., Roos K. P., Baskin R. J. Sarcomere length dispersion in single skeletal muscle fibers and fiber bundles. Biophys J. 1976 Aug;16(8):919–930. doi: 10.1016/S0006-3495(76)85742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome E. Relaxation of glycerinated muscle: low-angle x-ray diffraction studies. J Mol Biol. 1972 Mar 28;65(2):331–345. doi: 10.1016/0022-2836(72)90285-9. [DOI] [PubMed] [Google Scholar]
- Toylor D. L. Quantitative studies on the polarization optical properties of striated muscle. I. Birefringence changes of rabbit psoas muscle in the transition from rigor to relaxed state. J Cell Biol. 1976 Mar;68(3):497–511. doi: 10.1083/jcb.68.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zite-Ferenczy F., Rüdel R. A diffractometer using a lateral effect photodiode for the rapid determination of sarcomere length changes in cross-striated muscle. Pflugers Arch. 1978 Apr 25;374(1):97–100. doi: 10.1007/BF00585702. [DOI] [PubMed] [Google Scholar]


