Abstract
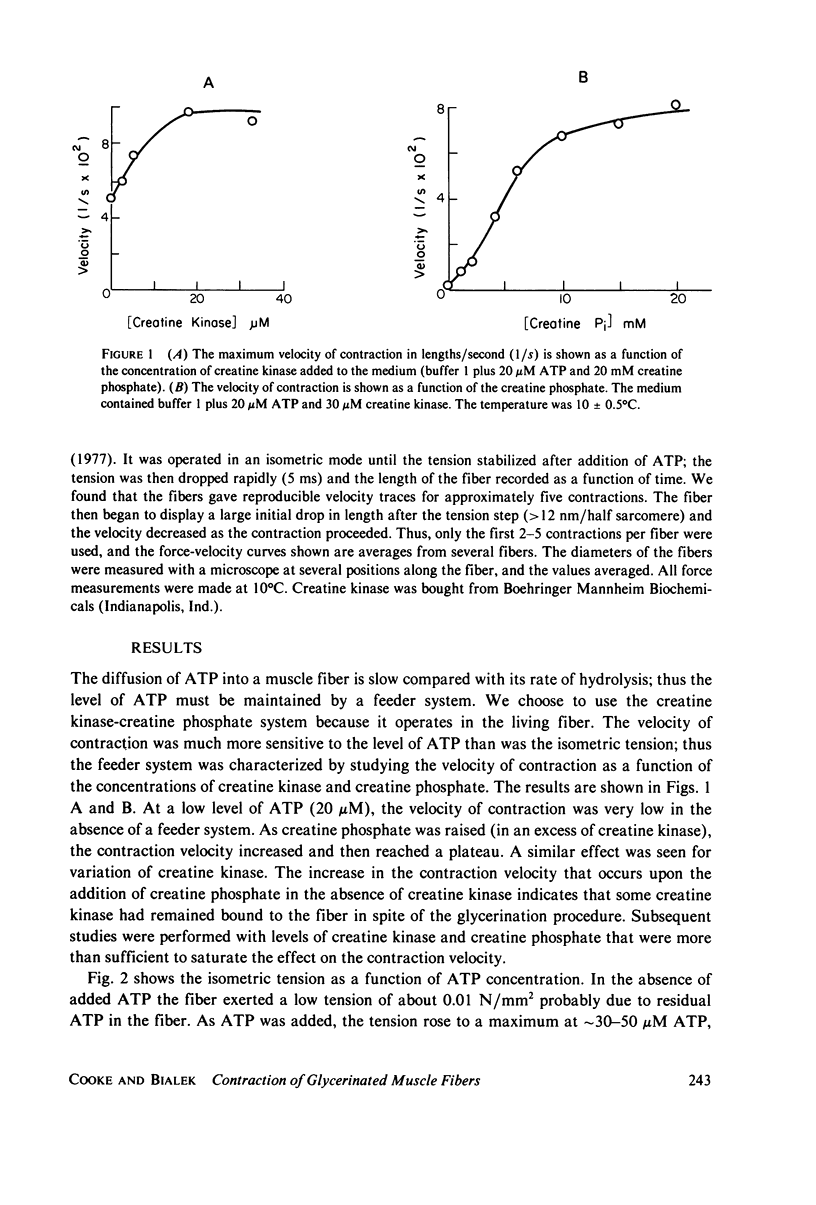
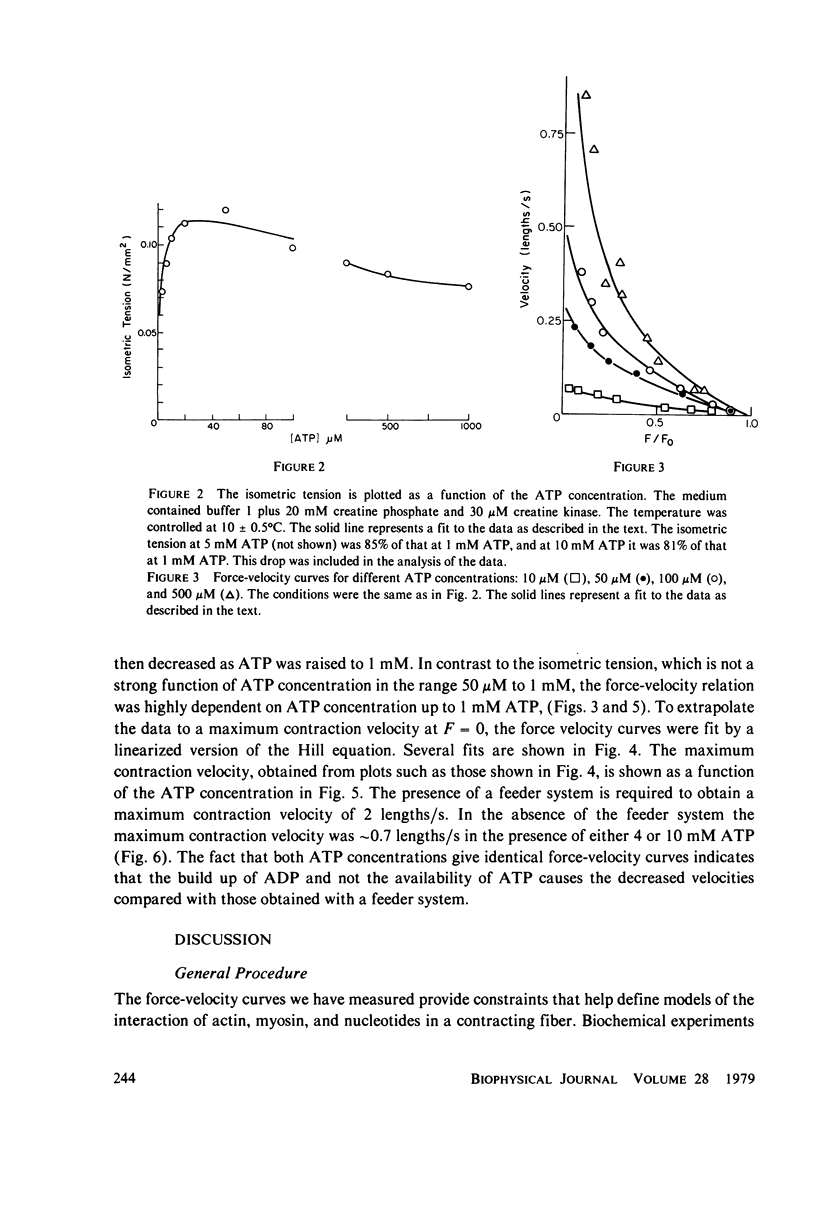
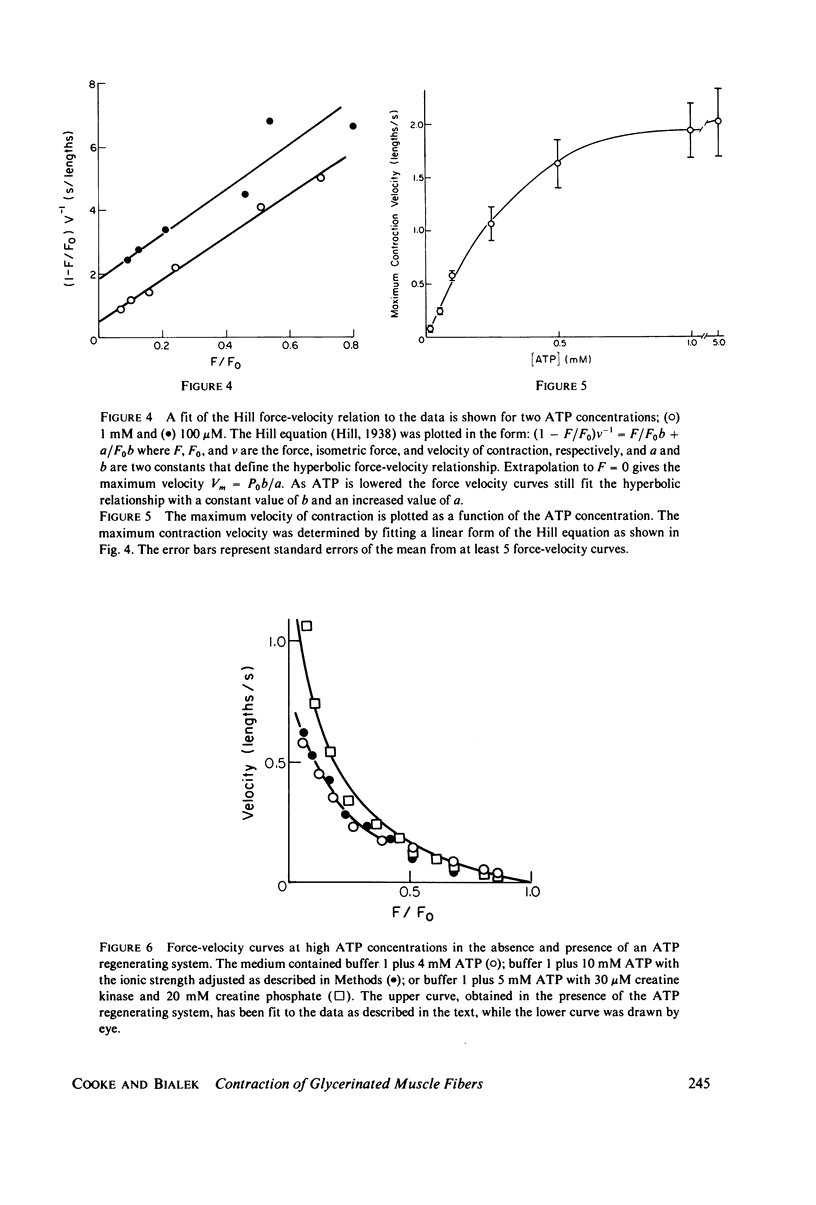
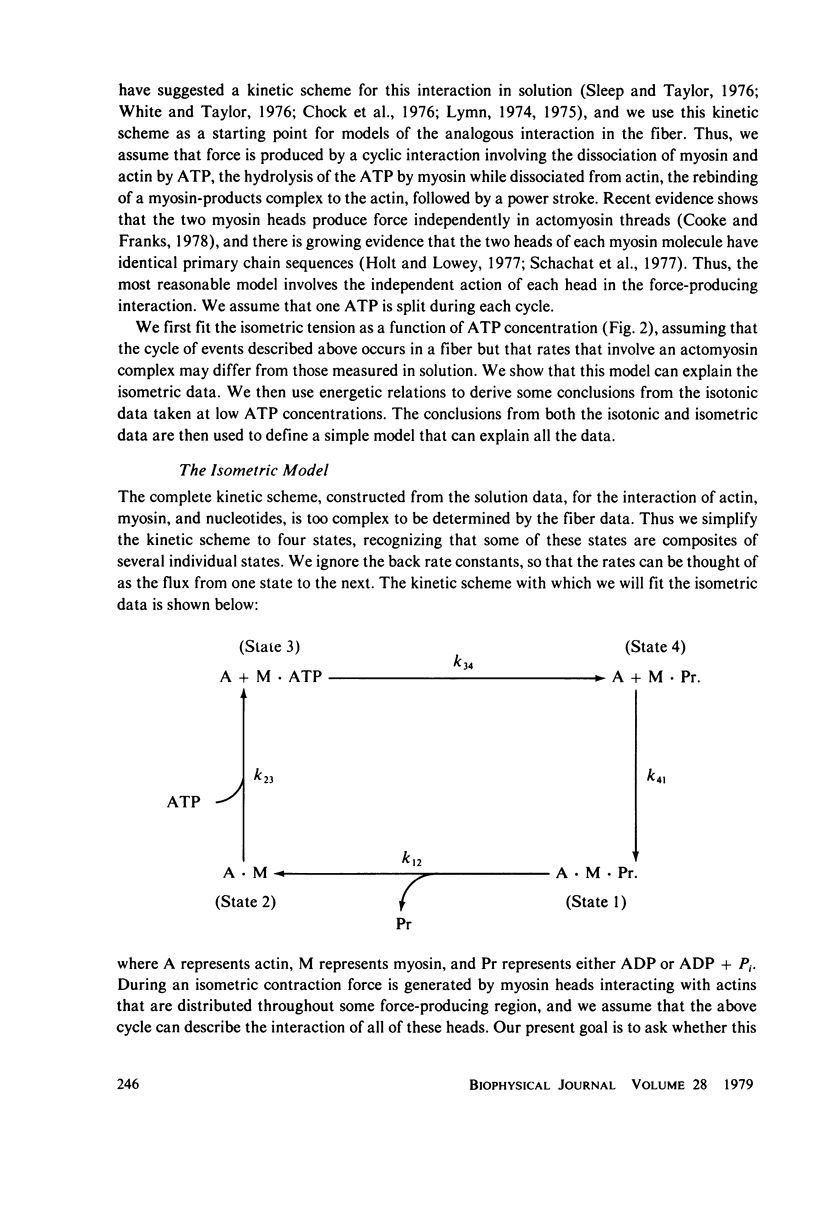
We have measured the force-velocity curves of glycerinated rabbit psoas fibers over a range of ATP concentration from 2.5 microM to 5 mM. As the ATP concentration is increased, the isometric tension increases to a maximum around 50 microM, then decreases to a plateau at 70% of the maximum by 1 mM ATP. At low ATP concentrations the maximum velocity of contraction is low and increases with increasing ATP, reaching a plateau at approximately 2 lengths per second by 1 mM ATP. Our studies suggest that the binding of ATP dissociates the myosin head from actin in the contracting muscle, a reaction similar to that seen in solution. We have constructed models of the actin-myosin-nucleotide interactions based on a kinetic scheme derived from solution studies. The fit of these models to the data shows that the rates of some reactions in the fiber must be considerably different from the rates of the analogous reactions in solution. The data is best fit by models in which head attachment occurs rapidly at the beginning of a power stroke, head detachment occurs rapidly at the end of the power stroke, and the force produced by a myosin head in a power stroke is independent of velocity.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arata T., Mukohata Y., Tonomura Y. Structure and function of the two heads of the myosin molecule. VI. ATP hydrolysis, shortening, and tension development of myofibrils. J Biochem. 1977 Sep;82(3):801–812. doi: 10.1093/oxfordjournals.jbchem.a131756. [DOI] [PubMed] [Google Scholar]
- Barden J. A., Mason P. Muscle crossbridge stroke and activity revealed by optical diffraction. Science. 1978 Mar 17;199(4334):1212–1213. doi: 10.1126/science.415364. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Effects of viscosity and ATP concentration on the movement of reactivated sea-urchin sperm flagella. J Exp Biol. 1975 Jun;62(3):701–719. doi: 10.1242/jeb.62.3.701. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J., Rintala D. Computer simulation of flagellar movement. V. oscillation of cross-bridge models with an ATP-concentration-dependent rate function. J Mechanochem Cell Motil. 1977 Sep;4(3):205–232. [PubMed] [Google Scholar]
- Chock S. P., Chock P. B., Eisenberg E. Pre-steady-state kinetic evidence for a cyclic interaction of myosin subfragment one with actin during the hydrolysis of adenosine 5'-triphosphate. Biochemistry. 1976 Jul 27;15(15):3244–3253. doi: 10.1021/bi00660a013. [DOI] [PubMed] [Google Scholar]
- Cooke R., Franks K. E. Generation of force by single-headed myosin. J Mol Biol. 1978 Apr 15;120(3):361–373. doi: 10.1016/0022-2836(78)90424-2. [DOI] [PubMed] [Google Scholar]
- Crooks R., Cooke R. Tension generation by threads of contractile proteins. J Gen Physiol. 1977 Jan;69(1):37–55. doi: 10.1085/jgp.69.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Gilbert C., Kretzschmar K. M., Wilkie D. R. The effect of the performance of work on total energy output and metabolism during muscular contraction. J Physiol. 1974 May;238(3):455–472. doi: 10.1113/jphysiol.1974.sp010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Hwang J. C. The force-velocity relationship in vertebrate muscle fibres at varied tonicity of the extracellular medium. J Physiol. 1977 Jul;269(2):255–272. doi: 10.1113/jphysiol.1977.sp011901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. A cross-bridge model of muscle contraction. Prog Biophys Mol Biol. 1978;33(1):55–82. doi: 10.1016/0079-6107(79)90025-7. [DOI] [PubMed] [Google Scholar]
- Fenn W. O. A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J Physiol. 1923 Dec 28;58(2-3):175–203. doi: 10.1113/jphysiol.1923.sp002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Homsher E., Simmons R. M., Trentham D. R. Reaction mechanism of the magnesium ion-dependent adenosine triphosphatase of frog muscle myosin and subfragment 1. Biochem J. 1978 Apr 1;171(1):165–175. doi: 10.1042/bj1710165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson B., Lymn R. W., Taylor E. W. Studies on the kinetics of formation and dissociation of the actomyosin complex. Biochemistry. 1969 Mar;8(3):811–819. doi: 10.1021/bi00831a008. [DOI] [PubMed] [Google Scholar]
- HILL A. V. THE EFFECT OF TENSION IN PROLONGING THE ACTIVE STATE IN A TWITCH. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:589–595. doi: 10.1098/rspb.1964.0021. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Chen Y. D., Podolsky R. J. Some self-consistent two-state sliding filament models of muscle contraction. Biophys J. 1975 Apr;15(4):335–372. doi: 10.1016/S0006-3495(75)85823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle. Part I. Prog Biophys Mol Biol. 1974;28:267–340. doi: 10.1016/0079-6107(74)90020-0. [DOI] [PubMed] [Google Scholar]
- Holt J. C., Lowey S. Distribution of alkali light chains in myosin: isolation of isoenzymes. Biochemistry. 1977 Oct 4;16(20):4398–4402. doi: 10.1021/bi00639a011. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Sollins K. R., Sollins M. R. A model for the transient and steady-state mechanical behavior of contracting muscle. Biophys J. 1974 Jul;14(7):546–562. doi: 10.1016/S0006-3495(74)85934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Two rigor states in skinned crayfish single muscle fibers. J Gen Physiol. 1976 Sep;68(3):267–280. doi: 10.1085/jgp.68.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M. Head rotation or dissociation? A study of exponential rate processes in chemically skinned rabbit muscle fibers when MgATP concentration is changed. Biophys J. 1978 Apr;22(1):97–103. doi: 10.1016/S0006-3495(78)85473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W. Actin activation of the myosin ATPase: a kinetic analysis. J Theor Biol. 1974 Feb;43(2):313–328. doi: 10.1016/s0022-5193(74)80063-9. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. The steady-state actomyosin ATPase: a further note. J Theor Biol. 1975 Feb;49(2):425–429. doi: 10.1016/0022-5193(75)90183-6. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Interaction of myosin subfragments with F-actin. Biochemistry. 1978 Dec 12;17(25):5431–5439. doi: 10.1021/bi00618a017. [DOI] [PubMed] [Google Scholar]
- Sleep J. A., Taylor E. W. Intermediate states of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5813–5817. doi: 10.1021/bi00671a019. [DOI] [PubMed] [Google Scholar]
- Takashi R., Putnam S. A fluorimetric method for continuously assaying ATPase: application to small specimens of glycerol-extracted muscle fibers. Anal Biochem. 1979 Jan 15;92(2):375–382. doi: 10.1016/0003-2697(79)90674-2. [DOI] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]



