Abstract
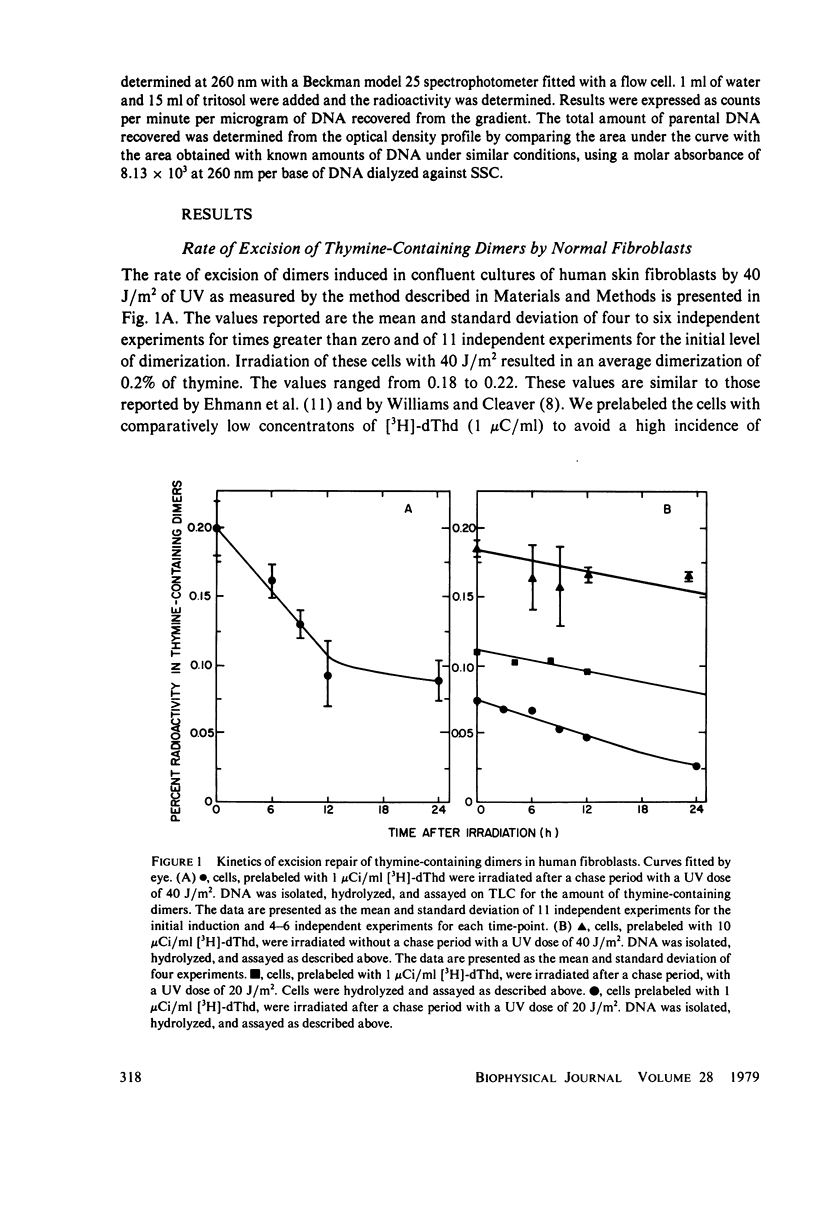
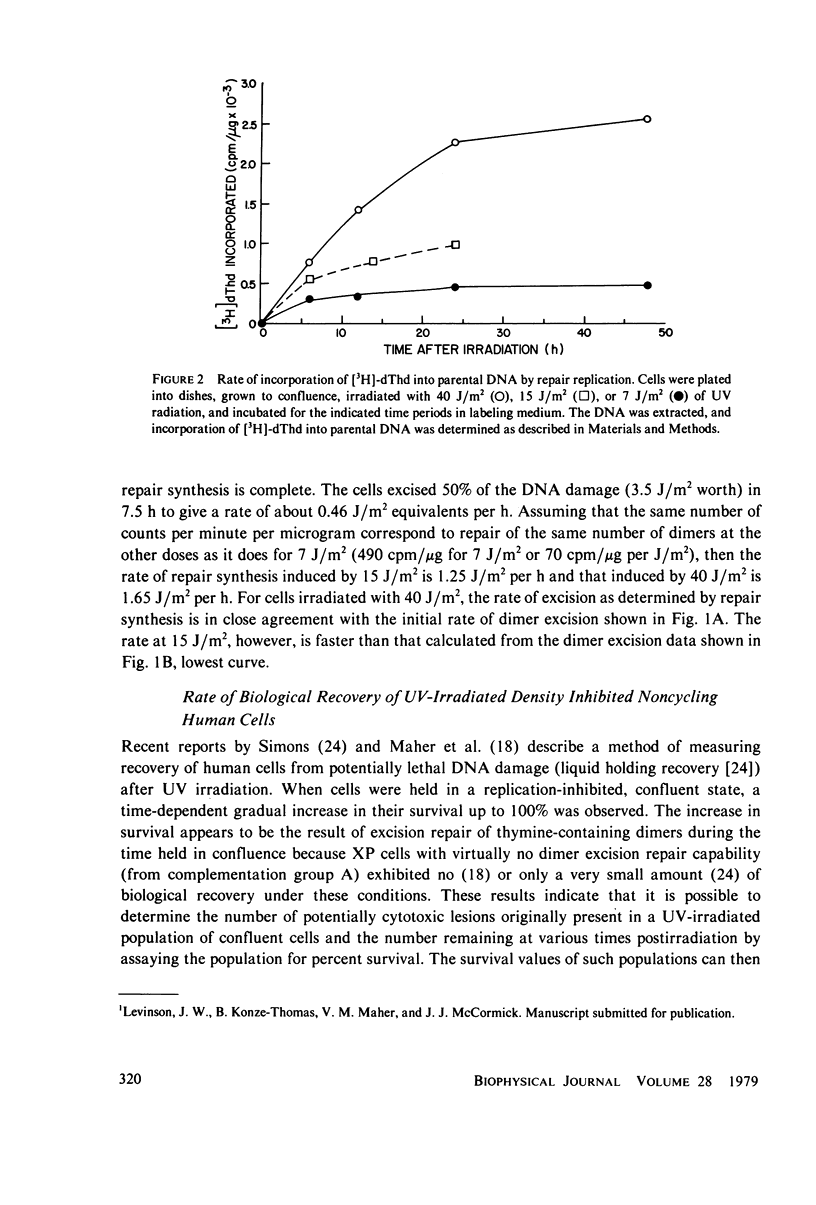
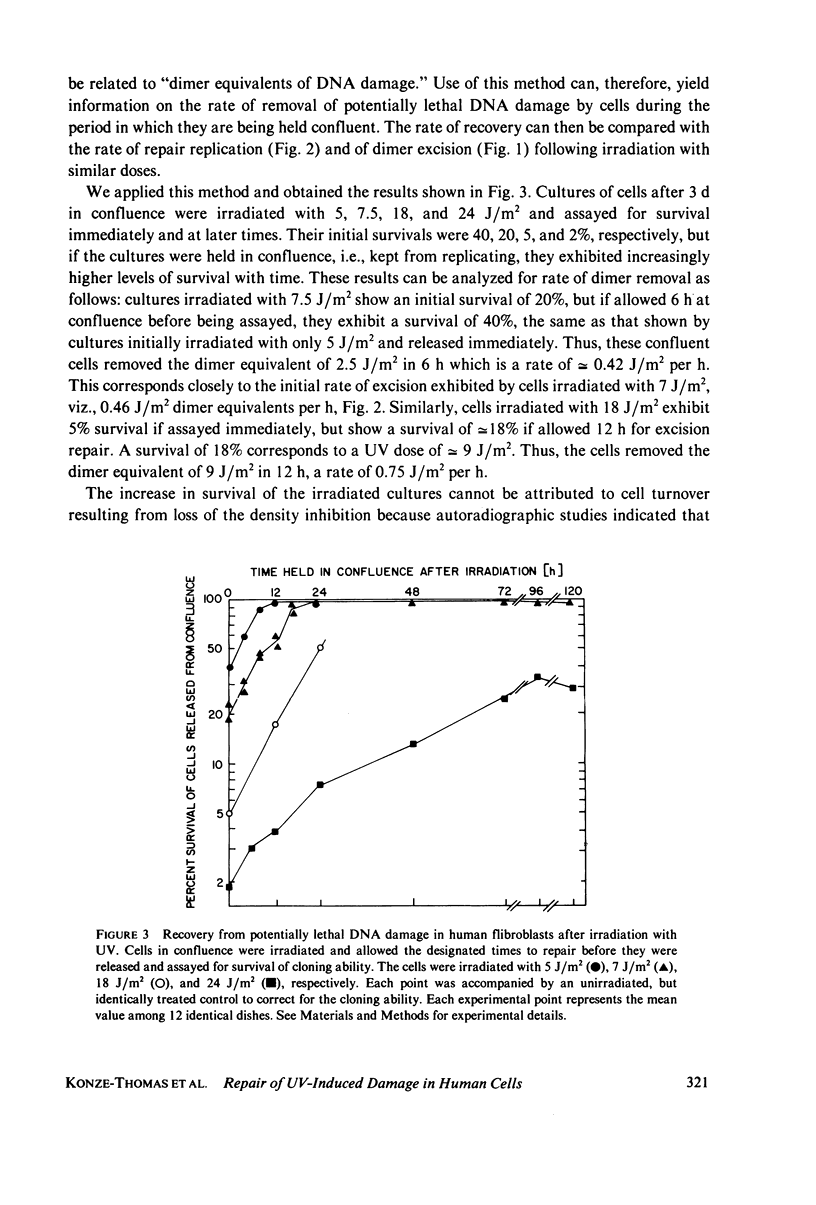
The kinetics of excision repair in confluent cultures of diploid human fibroblasts after ultraviolet irradiation at varying doses was measured by three different methods: (a) removal of thymine-containing dimers, (b) DNA excision repair synthesis, and (c) biological recovery of cells from the potentially lethal effects of the irradiation. Each method gave similar results and indicated that the excision rate was dependent upon the number of thymine-containing dimers induced (substrate concentration). For example, at a dose of 40 J/m2 (0.2% dimerization), the repair rate was 1.6 J/m2 per h as determined by a modified method to measure the number of thymine-containing dimers remaining in DNA and 1.65 J/m2 as measured by excision repair synthesis. At a dose of 7.5 J/m2, the repair rate was 0.5 J/m2 per h as measured by biological recovery, and at a dose of 7 J/m2, the repair rate was 0.46 J/m2 per h as measured by excision repair synthesis.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed F. E., Setlow R. B. Excision repair in ataxia telangiectasia, Fanconi's anemia, Cockayne syndrome, and Bloom's syndrome after treatment with ultraviolet radiation and N-acetoxy-2-acetylaminofluorene. Biochim Biophys Acta. 1978 Dec 21;521(2):805–817. doi: 10.1016/0005-2787(78)90319-2. [DOI] [PubMed] [Google Scholar]
- Amacher D. E., Elliott J. A., Lieberman M. W. Differences in removal of acetylaminofluorene and pyrimidine dimers from the DNA of cultured mammalian cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1553–1557. doi: 10.1073/pnas.74.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk P. G., Lutzner M. A., Clarke D. D., Robbins J. H. Ultraviolet-stimulated thymidine incorporation in xeroderma pigmentosum lymphocytes. J Lab Clin Med. 1971 May;77(5):759–767. [PubMed] [Google Scholar]
- Clarkson J. M., Evans H. J. Unscheduled DNA synthesis in human leucocytes after exposure to UV light, -rays and chemical mutagens. Mutat Res. 1972 Apr;14(4):413–430. doi: 10.1016/0027-5107(72)90139-x. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Induction of thioguanine- and ouabain-resistant mutants and single-strand breaks in the DNA of Chinese hamster ovary cells by 3H-thymidine. Genetics. 1977 Sep;87(1):129–138. doi: 10.1093/genetics/87.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor W. G., Norman A. Unscheduled DNA synthesis in human leucocytes. Mutat Res. 1971 Dec;13(4):393–402. doi: 10.1016/0027-5107(71)90051-0. [DOI] [PubMed] [Google Scholar]
- Cook K. H., Friedberg E. C. Measurement of thymine dimers in DNA by thin-layer chromatography. II. The use of one-dimensional systems. Anal Biochem. 1976 Jun;73(2):411–418. doi: 10.1016/0003-2697(76)90188-3. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Hanawalt P. C. The timecourse of DNA repair replication in ultraviolet-irradiated HeLa cells. Biochim Biophys Acta. 1973 Oct 12;324(2):206–217. doi: 10.1016/0005-2787(73)90138-x. [DOI] [PubMed] [Google Scholar]
- Ehmann U. K., Cook K. H., Friedberg E. C. The kinetics of thymine dimer excision in ultraviolet-irradiated human cells. Biophys J. 1978 May;22(2):249–264. doi: 10.1016/S0006-3495(78)85487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Birch N., Otto J. R., MacCormick J. J. Cytotoxicity of carcinogenic aromatic amides in normal and xeroderma pigmentosum fibroblasts with different DNA repair capabilities. J Natl Cancer Inst. 1975 Jun;54(6):1287–1294. doi: 10.1093/jnci/54.6.1287. [DOI] [PubMed] [Google Scholar]
- Maher V. M., McCormick J. J., Grover P. L., Sims P. Effect of DNA repair on the cytotoxicity and mutagenicity of polycyclic hydrocarbon derivatives in normal and xeroderma pigmentosum human fibroblasts. Mutat Res. 1977 Apr;43(1):117–138. doi: 10.1016/0027-5107(77)90137-3. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Cleaver J. E. Repair replication in HeLa cells after large doses of x-irradiation. Nature. 1967 Oct 28;216(5113):369–370. doi: 10.1038/216369a0. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Lohman P. H., Sluyter M. L. Use of UV endonuclease from Micrococcus luteus to monitor the progress of DNA repair in UV-irradiated human cells. Mutat Res. 1973 Aug;19(2):245–256. doi: 10.1016/0027-5107(73)90083-3. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Trosko J. E., Carrier W. L. Evidence for excision of ultraviolet-induced pyrimidine dimers from the DNA of human cells in vitro. Biophys J. 1968 Mar;8(3):319–325. doi: 10.1016/S0006-3495(68)86490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D., German J., Carrier W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J. W. Development of a liquid-holding technique for the study of DNA-repair in human diploid fibroblasts. Mutat Res. 1979 Feb;59(2):273–283. doi: 10.1016/0027-5107(79)90165-9. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Hanawalt P. C. Repair replication in cultured normal and transformed human fibroblasts. Biochim Biophys Acta. 1976 Oct 4;447(2):121–132. doi: 10.1016/0005-2787(76)90335-x. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Sekiguchi M., Okada Y. Restoration of ultraviolet-induced unscheduled DNA synthesis of xeroderma pigmentosum cells by the concomitant treatment with bacteriophage T4 endonuclease V and HVJ (Sendai virus). Proc Natl Acad Sci U S A. 1975 Oct;72(10):4071–4075. doi: 10.1073/pnas.72.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichselbaum R. R., Nove J., Little J. B. Deficient recovery from potentially lethal radiation damage in ataxia telengiectasia and xeroderma pigmentosum. Nature. 1978 Jan 19;271(5642):261–262. doi: 10.1038/271261a0. [DOI] [PubMed] [Google Scholar]
- Williams J. I., Cleaver J. E. Excision repair of ultraviolet damage in mammalian cells. Evidence for two steps in the excision of pyrimidine dimers. Biophys J. 1978 May;22(2):265–279. doi: 10.1016/S0006-3495(78)85488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]



