Abstract
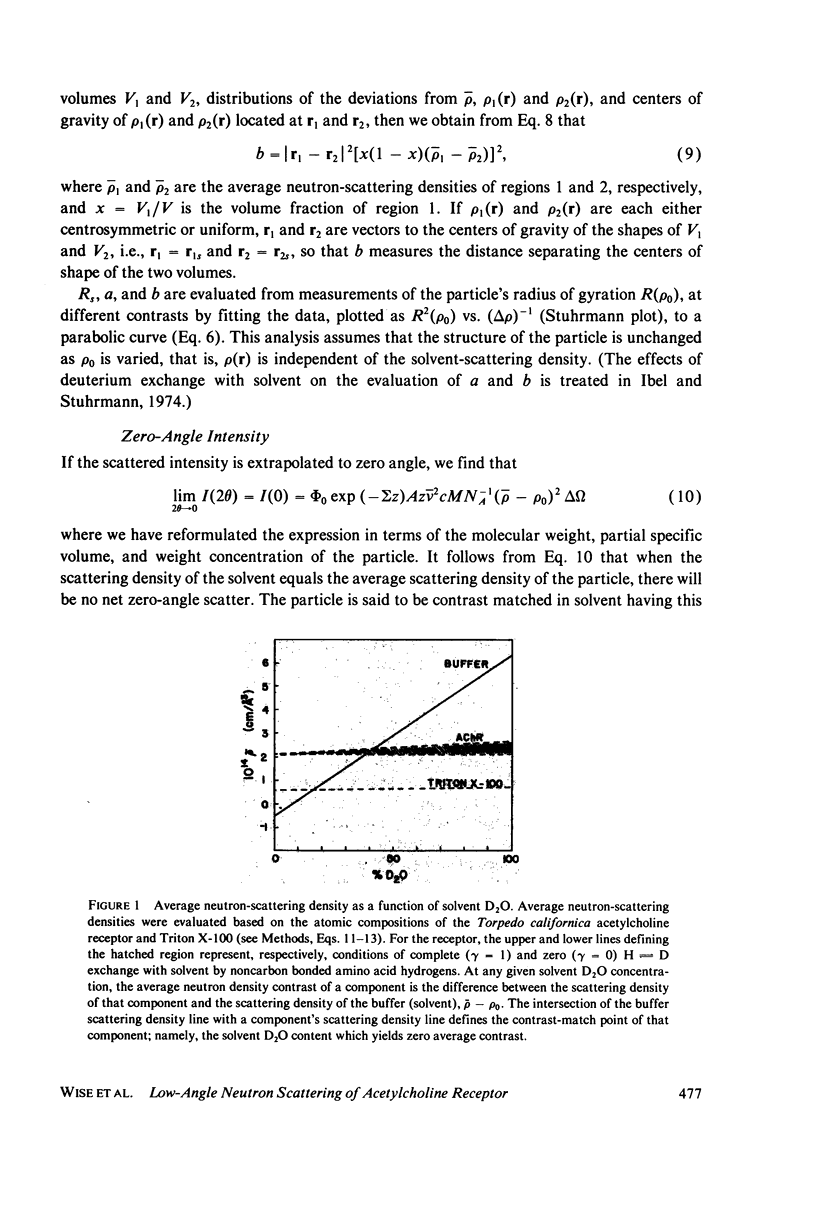
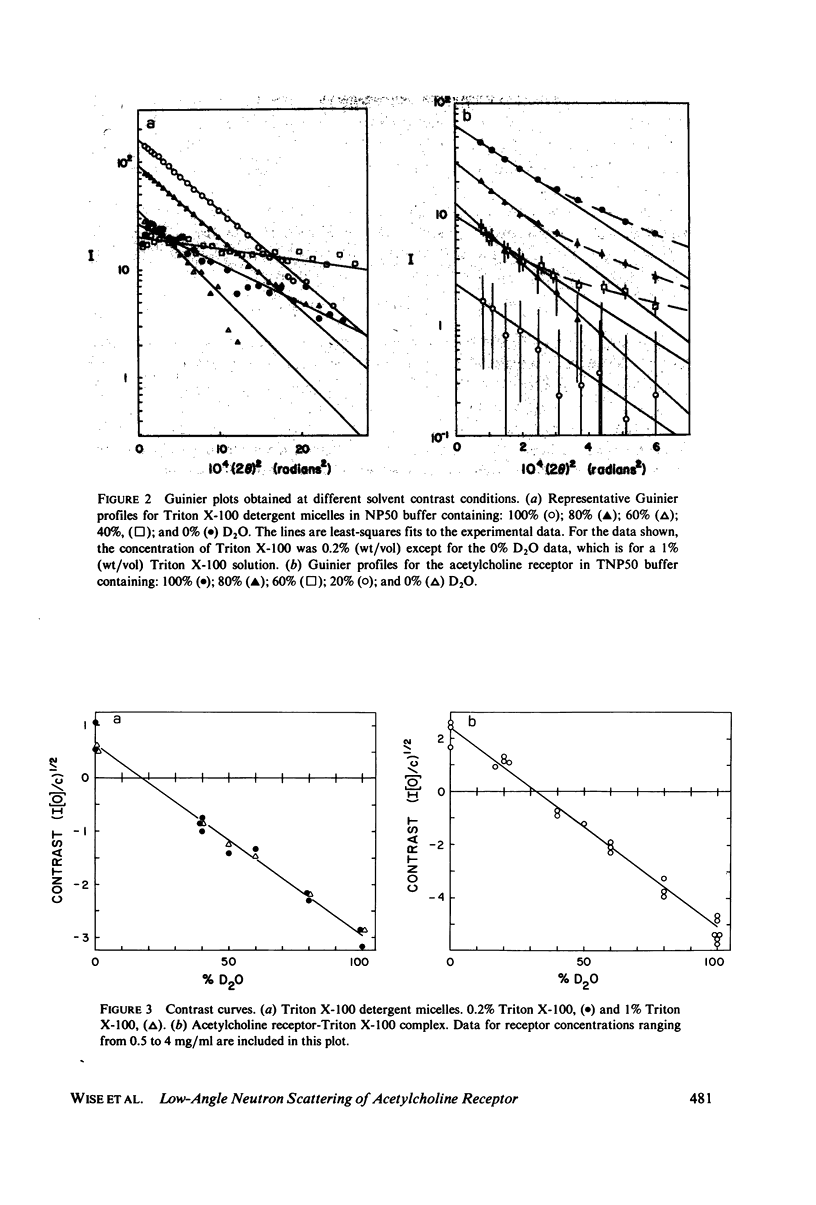
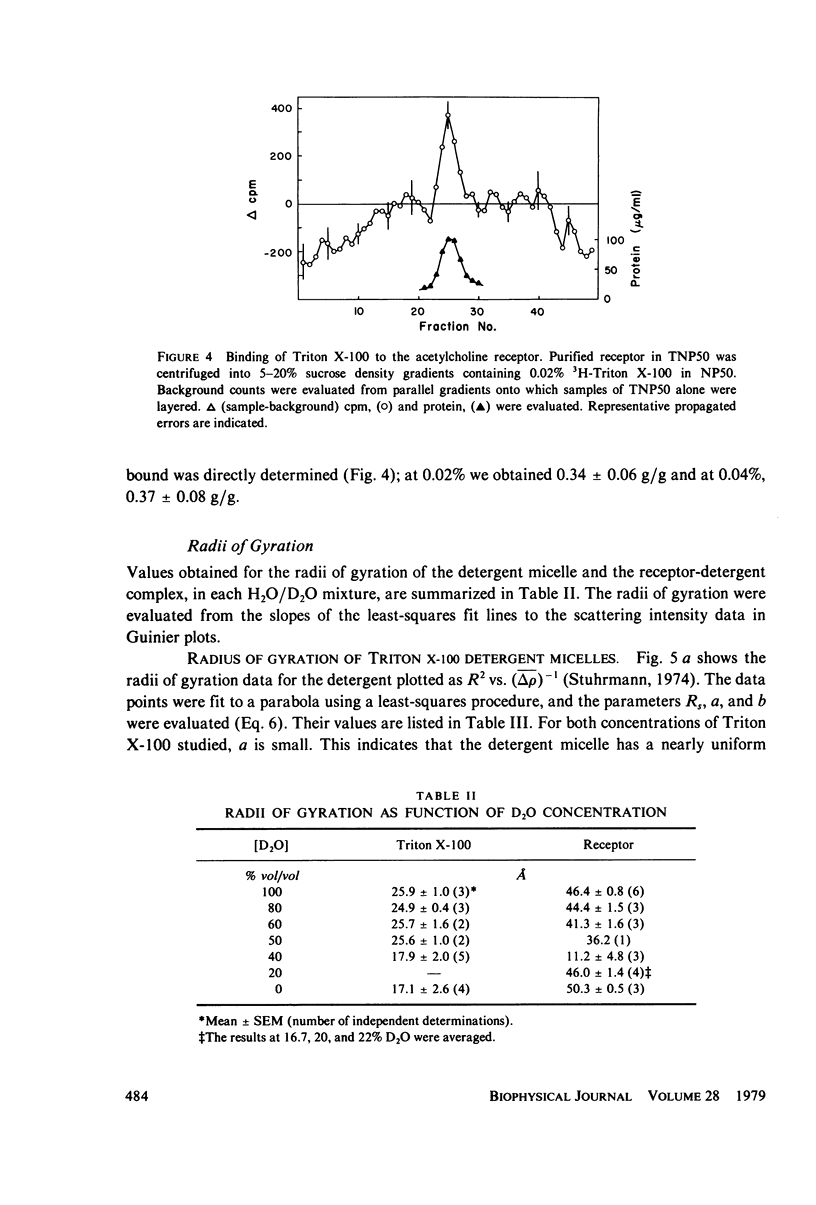
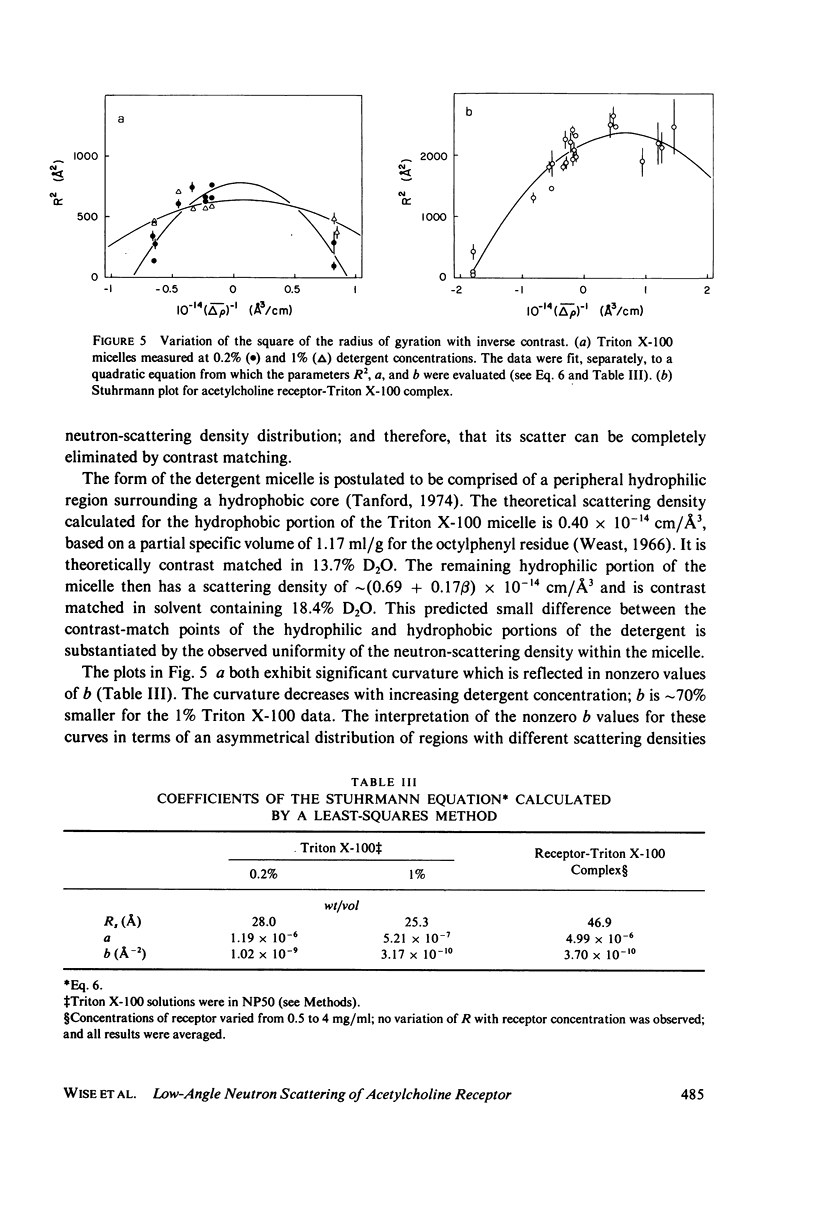
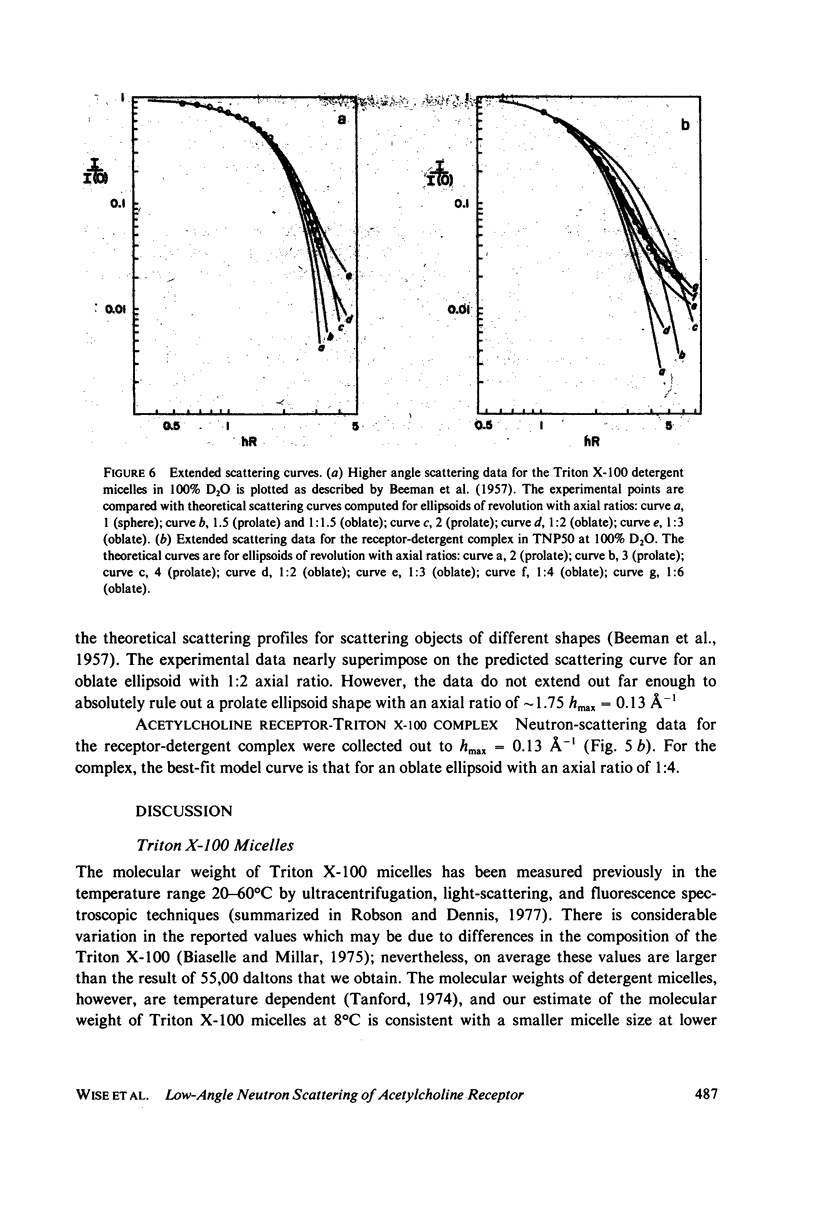
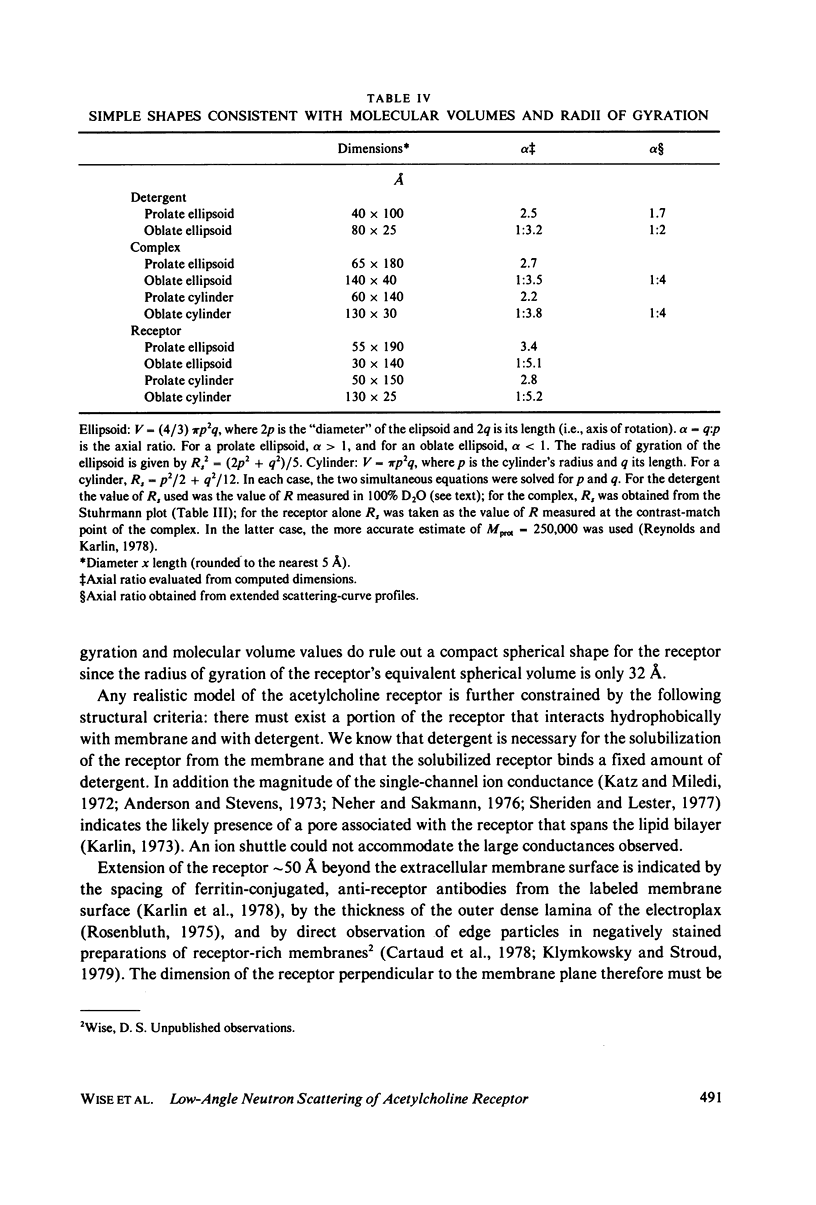
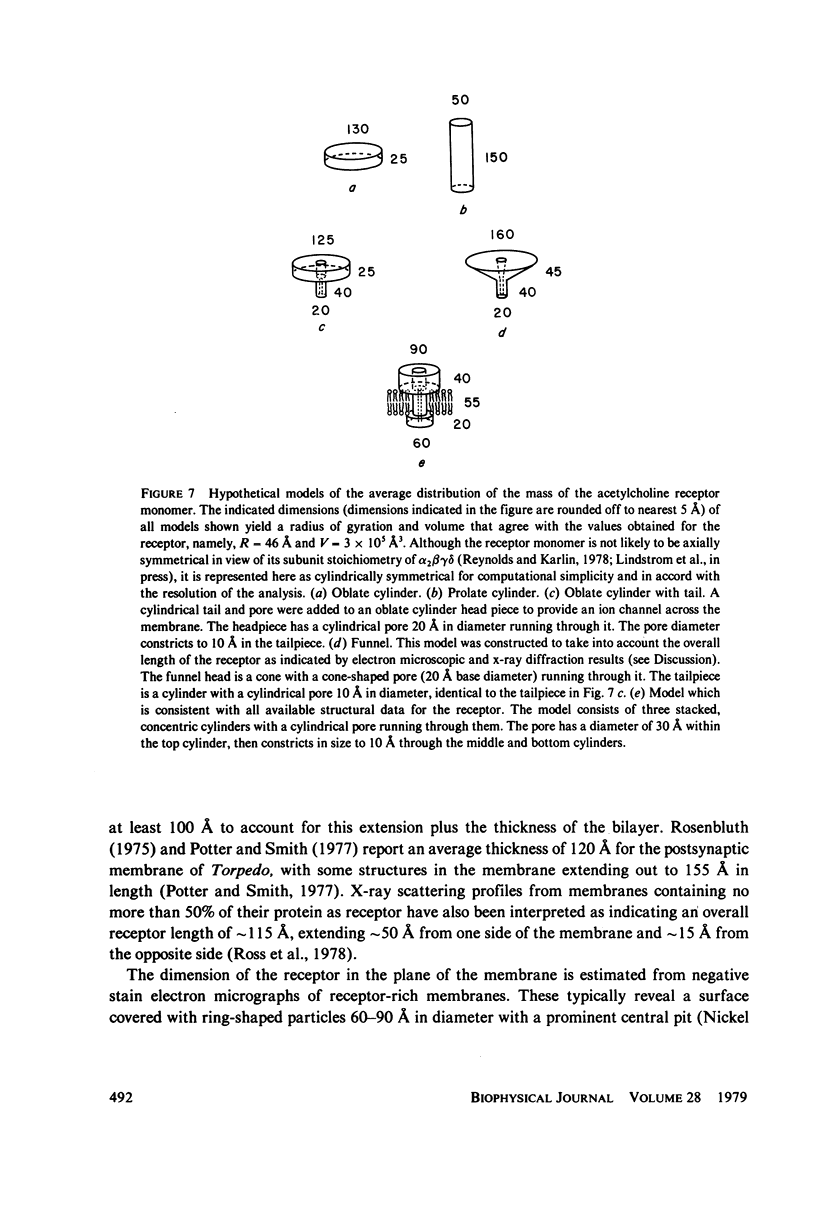
The acetylcholine receptor from the electric tissue of Torpedo californica is a large, integral membrane protein containing four different types of polypeptide chains. The structure of the purified receptor in detergent solution has previously been investigated by sedimentation analysis and gel filtration. Sedimentation analysis yielded a molecular weight of 250,000 for the protein moiety of the receptor monomer-detergent complex; hydrodynamic characteristics such as the Stokes radius, however, refer to the receptor-detergent complex. In this paper we report the results of our use of low-angle neutron scattering to investigate the shape of the receptor-detergent (Triton X-100 from Rohm & Haas Co., Philadelphia, Pa.) complex and separately of its protein and detergent moieties. By adjustment of the neutron-scattering density of the solvent with D2O to match that of one or the other of the moieties, its contribution to the scattering can be nearly, if not completely, eliminated. Neutron scattering from Triton X-100 micelles established that this detergent is contrast matched in approximately 18% D2O. Scattering measurements on the receptor-detergent complex in this solvent yielded a radius of gyration of the acetylcholine receptor monomer of 46 +/- 1A. The radius of gyration and molecular volume (305,000 A3) of the receptor are inconsistent with a compact spherical shape. These parameters are consistent with, for example, a prolate cylinder of dimensions (length x diameter) approximately 150 x approximately 50 A or an oblate cylinder, approximately 25 x approximately 130 A. More complex shapes are possible and in fact seem to be required to reconcile the present results with previous electron microscopic and x-ray analyses of receptor in membrane and with considerations of the function of the receptor in controlling ion permeability. The neutron-scattering data yield, in addition, an independent determination of the molecular weight of the receptor protein (240,000 +/- 40,000), the extent of Triton X-100 binding in the complex (approximately 0.4 g/g protein), and from the extended scattering curve, an approximation to the shape of the receptor-Triton X-100 complex, namely an oblate ellipsoid of axial ratio 1:4.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biaselle C. J., Millar D. B. Studies on Triton X-100 detergent micelles. Biophys Chem. 1975 Oct;3(4):355–361. doi: 10.1016/0301-4622(75)80029-9. [DOI] [PubMed] [Google Scholar]
- Carroll R. C., Eldefrawi M. E., Edelstein S. J. Studies on the structure of the acetylcholine receptor from Torpedo marmorata. Biochem Biophys Res Commun. 1973 Dec 10;55(3):864–872. doi: 10.1016/0006-291x(73)91224-2. [DOI] [PubMed] [Google Scholar]
- Cartaud J., Benedetti E. L. A morphological study of the cholinergic receptor protein from Torpedo marmorata in its membrane environment and in its detergent-extracted purified form. J Cell Sci. 1978 Feb;29:313–337. doi: 10.1242/jcs.29.1.313. [DOI] [PubMed] [Google Scholar]
- Cartaud J., Benedetti E. L., Cohen J. B., Meunier J. C., Changeux J. P. Presence of a lattice structure in membrane fragments rich in nicotinic receptor protein from the electric organ of Torpedo marmorata. FEBS Lett. 1973 Jun 15;33(1):109–113. doi: 10.1016/0014-5793(73)80171-1. [DOI] [PubMed] [Google Scholar]
- Chang H. W., Bock E. Molecular forms of acetylcholine receptor. Effects of calcium ions and a sulfhydryl reagent on the occurrence of oligomers. Biochemistry. 1977 Oct 4;16(20):4513–4520. doi: 10.1021/bi00639a028. [DOI] [PubMed] [Google Scholar]
- Chang H. W., Bock E. Structural stabilization of isolated acetylcholine receptor: specific interaction with phospholipids. Biochemistry. 1979 Jan 9;18(1):172–179. doi: 10.1021/bi00568a026. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Potter L. T., Smith D. S. Postsynaptic membranes in the electric tissue of Narcine: IV. Isolation and characterization of the nicotinic receptor protein. Tissue Cell. 1977;9(4):623–644. doi: 10.1016/0040-8166(77)90031-3. [DOI] [PubMed] [Google Scholar]
- Clarke S. The size and detergent binding of membrane proteins. J Biol Chem. 1975 Jul 25;250(14):5459–5469. [PubMed] [Google Scholar]
- Damle V. N., Karlin A. Affinity labeling of one of two alpha-neurotoxin binding sites in acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2039–2045. doi: 10.1021/bi00604a002. [DOI] [PubMed] [Google Scholar]
- Dupont Y., Cohen J. B., Changeux J. P. X-ray diffraction study of membrane fragments rich in acetylcholine receptor protein prepared from the electric organ of Torpedo marmorata. FEBS Lett. 1974 Mar 15;40(1):130–133. doi: 10.1016/0014-5793(74)80910-5. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J., Beyer W. B., Elderfrawi A. T., Elderfrawi M. E. Molecular weight of the acetylcholine receptors of electric organs and the effect of Triton X-100. J Biol Chem. 1975 Aug 10;250(15):6101–6106. [PubMed] [Google Scholar]
- Eldefrawi M. E., Eldefrawi A. T., Wilson D. B. Tryptophan and cystein residues of the acetylcholine receptors of Torpedo species. Relationship to binding of cholinergic ligands. Biochemistry. 1975 Sep 23;14(19):4304–4310. doi: 10.1021/bi00690a026. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Moore P. B. Determination of quaternary structure by small angle neutron scattering. Annu Rev Biophys Bioeng. 1975;4(00):219–241. doi: 10.1146/annurev.bb.04.060175.001251. [DOI] [PubMed] [Google Scholar]
- Gibson R. E., O'Brien R. D., Edelstein S. J., Thompson W. R. Acetylcholine receptor oligomers from electroplax of Torpedo species. Biochemistry. 1976 Jun 1;15(11):2377–2383. doi: 10.1021/bi00656a020. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., McLaughlin M., Karlin A. Disulfide bond cross-linked dimer in acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1977 Dec 7;79(3):692–699. doi: 10.1016/0006-291x(77)91167-6. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., McLaughlin M., Karlin A. Formation of disulfide-linked oligomers of acetylcholine receptor in membrane from torpedo electric tissue. Biochemistry. 1979 Jan 9;18(1):155–163. doi: 10.1021/bi00568a024. [DOI] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Structural and functional properties of the acetylcholine receptor protein in its purified and membrane-bound states. Annu Rev Biochem. 1978;47:317–357. doi: 10.1146/annurev.bi.47.070178.001533. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Ibel K. Comparison of neutron and X-ray scattering of dilute myoglobin solutions. J Mol Biol. 1975 Apr 5;93(2):255–265. doi: 10.1016/0022-2836(75)90131-x. [DOI] [PubMed] [Google Scholar]
- Karlin A., Holtzman E., Valderrama R., Damle V., Hsu K., Reyes F. Binding of antibodies to acetylcholine receptors in Electrophorus and Torpedo electroplax membranes. J Cell Biol. 1978 Mar;76(3):577–592. doi: 10.1083/jcb.76.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A. Molecular interactions of the acetylcholine receptor. Fed Proc. 1973 Aug;32(8):1847–1853. [PubMed] [Google Scholar]
- Karlin A., Weill C. L., McNamee M. G., Valderrama R. Facets of the structures of acetylcholine receptors from Electrophorus and Torpedo. Cold Spring Harb Symp Quant Biol. 1976;40:203–210. doi: 10.1101/sqb.1976.040.01.022. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky M. W., Stroud R. M. Immunospecific identification and three-dimensional structure of a membrane-bound acetylcholine receptor from Torpedo californica. J Mol Biol. 1979 Mar 5;128(3):319–334. doi: 10.1016/0022-2836(79)90091-3. [DOI] [PubMed] [Google Scholar]
- Lewis M. S., Krieg L. C., Kirk W. D. The molecular weight and detergent binding of bovine rhodopsin. Exp Eye Res. 1974 Jan;18(1):29–40. doi: 10.1016/0014-4835(74)90041-4. [DOI] [PubMed] [Google Scholar]
- Luzzati V., Tardieu A., Mateu L. Structure of human serum lipoproteins in solution. I. Theory and techniques of an x-ray scattering approach using solvents of variable density. J Mol Biol. 1976 Feb 25;101(2):115–127. doi: 10.1016/0022-2836(76)90367-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Carrion M., Sator V., Raftery M. A. The molecular weight of an acetylcholine receptor isolated from Torpedo californica. Biochem Biophys Res Commun. 1975 Jul 8;65(1):129–137. doi: 10.1016/s0006-291x(75)80070-2. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Olsen R. W., Changeux J. P. Studies on the cholinergic receptor protein from Electrophorus electricus. Effect of detergents on some hydrodynamic properties of the receptor protein in solution. FEBS Lett. 1972 Jul 15;24(1):63–68. doi: 10.1016/0014-5793(72)80827-5. [DOI] [PubMed] [Google Scholar]
- Moody T., Schmidt J., Raftery M. A. Binding of acetylcholine and related compounds to purified acetylcholine receptor from Torpedo Californica electroplax. Biochem Biophys Res Commun. 1973 Aug 6;53(3):761–772. doi: 10.1016/0006-291x(73)90158-7. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Langer J. A., Schoenborn B. P., Engelman D. M. Triangulation of proteins in the 30 S ribosomal subunit of Exherichia coli. J Mol Biol. 1977 May 15;112(2):199–227. doi: 10.1016/s0022-2836(77)80139-3. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Nickel E., Potter L. T. Ultrastructure of isolated membranes of Torpedo electric tissue. Brain Res. 1973 Jul 27;57(2):508–517. doi: 10.1016/0006-8993(73)90158-3. [DOI] [PubMed] [Google Scholar]
- O'Brien R. D., Gibson R. E. Conversion of high affinity acetylcholine receptor from Torpedo californica electroplax to an altered form. Arch Biochem Biophys. 1975 Aug;169(2):458–463. doi: 10.1016/0003-9861(75)90188-5. [DOI] [PubMed] [Google Scholar]
- Osborne H. B., Sardet C., Helenius A. Bovine rhodopsin: characterization of the complex formed with Triton X-100. Eur J Biochem. 1974 May 15;44(2):383–390. doi: 10.1111/j.1432-1033.1974.tb03495.x. [DOI] [PubMed] [Google Scholar]
- Osborne H. B., Sardet C., Michel-Villaz M., Chabre M. Structural study of rhodopsin in detergent micelles by small-angle neutron scattering. J Mol Biol. 1978 Aug 5;123(2):177–206. doi: 10.1016/0022-2836(78)90320-0. [DOI] [PubMed] [Google Scholar]
- Potter L. T., Smith D. S. Postsynaptic membranes in the electric tissue of Narcine: I. Organization and innervation of electric cells. Fine structure of nicotinic receptor-channel molecules revealed by transmission microscopy. Tissue Cell. 1977;9(4):585–594. doi: 10.1016/0040-8166(77)90028-3. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Schmidt J., Clark D. G. Specificity of -bungarotoxin binding to Torpedo californica electroplax. Arch Biochem Biophys. 1972 Oct;152(2):882–886. doi: 10.1016/0003-9861(72)90285-8. [DOI] [PubMed] [Google Scholar]
- Reed K., Vandlen R., Bode J., Duguid J., Raftery M. A. Characterization of acetylcholine receptor-rich and acetylcholinesterase-rich membrane particles from Torpedo californica electroplax. Arch Biochem Biophys. 1975 Mar;167(1):138–144. doi: 10.1016/0003-9861(75)90449-x. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Karlin A. Molecular weight in detergent solution of acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2035–2038. doi: 10.1021/bi00604a001. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Determination of molecular weight of the protein moiety in protein-detergent complexes without direct knowledge of detergent binding. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4467–4470. doi: 10.1073/pnas.73.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. C., Tanford C. The binding of deoxycholate, Triton X-100, sodium dodecyl sulfate, and phosphatidylcholine vesicles to cytochrome b5. Biochemistry. 1975 Jan 28;14(2):369–378. doi: 10.1021/bi00673a025. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Synaptic membrane structure in Torpedo electric organ. J Neurocytol. 1975 Dec;4(6):697–712. doi: 10.1007/BF01181631. [DOI] [PubMed] [Google Scholar]
- Ross M. J., Klymkowsky M. W., Agard D. A., Stroud R. M. Structural studies of a membrane-bound acetylcholine receptor from Torpedo californica. J Mol Biol. 1977 Nov;116(4):635–659. doi: 10.1016/0022-2836(77)90264-9. [DOI] [PubMed] [Google Scholar]
- Sardet C., Tardieu A., Luzzati V. Shape and size of bovine rhodopsin: a small-angle x-ray scattering study of a rhodopsin-detergent complex. J Mol Biol. 1976 Aug 15;105(3):383–407. doi: 10.1016/0022-2836(76)90100-5. [DOI] [PubMed] [Google Scholar]
- Schoenborn B. P. Neutron scattering for the analysis of membranes. Biochim Biophys Acta. 1976 Apr 13;457(1):41–55. doi: 10.1016/0304-4157(76)90013-7. [DOI] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Rates and equilibria at the acetylcholine receptor of Electrophorus electroplaques: a study of neurally evoked postsynaptic currents and of voltage-jump relaxations. J Gen Physiol. 1977 Aug;70(2):187–219. [PMC free article] [PubMed] [Google Scholar]
- Suarez-Isla B. A., Hucho F. Acetylcholine receptor: SH group reactivity as indicator of conformational changes and functional states. FEBS Lett. 1977 Mar 15;75(1):65–69. doi: 10.1016/0014-5793(77)80054-9. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Changeux J. P. Interconversion between different states of affinity for acetylcholine of the cholinergic receptor protein from Torpedo marmorata. Eur J Biochem. 1975 Jul 15;55(3):505–515. doi: 10.1111/j.1432-1033.1975.tb02188.x. [DOI] [PubMed] [Google Scholar]
- Tanford C., Nozaki Y., Reynolds J. A., Makino S. Molecular characterization of proteins in detergent solutions. Biochemistry. 1974 May 21;13(11):2369–2376. doi: 10.1021/bi00708a021. [DOI] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Weill C. L., McNamee M. G., Karlin A. Affinity-labeling of purified acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1974 Dec 11;61(3):997–1003. doi: 10.1016/0006-291x(74)90254-x. [DOI] [PubMed] [Google Scholar]


