Abstract
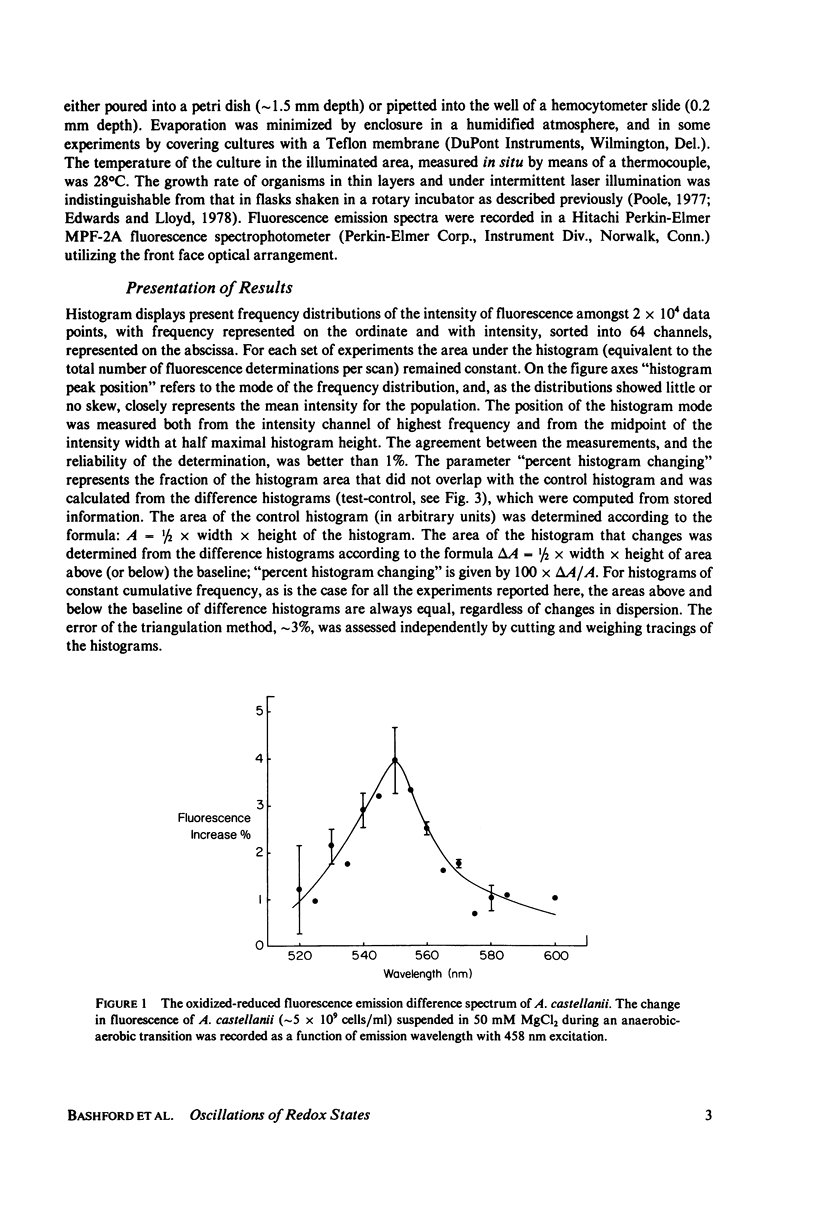
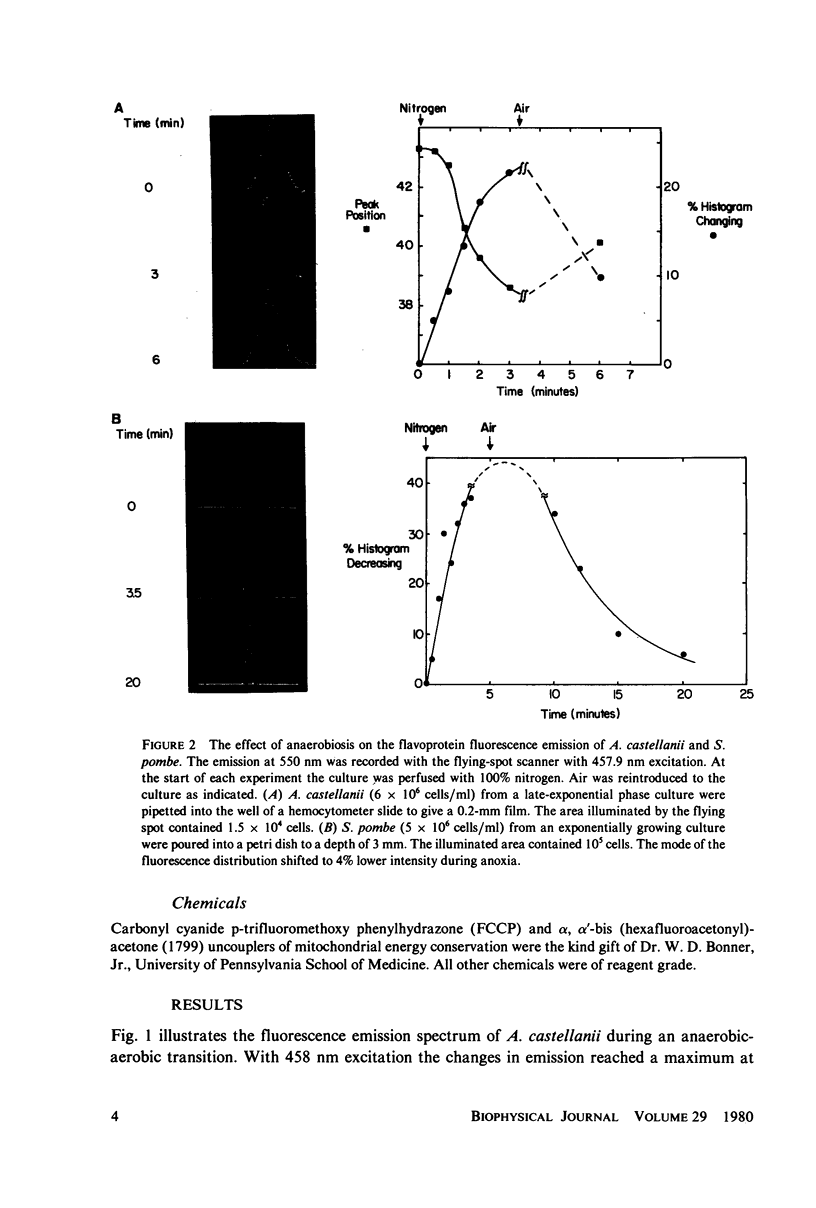
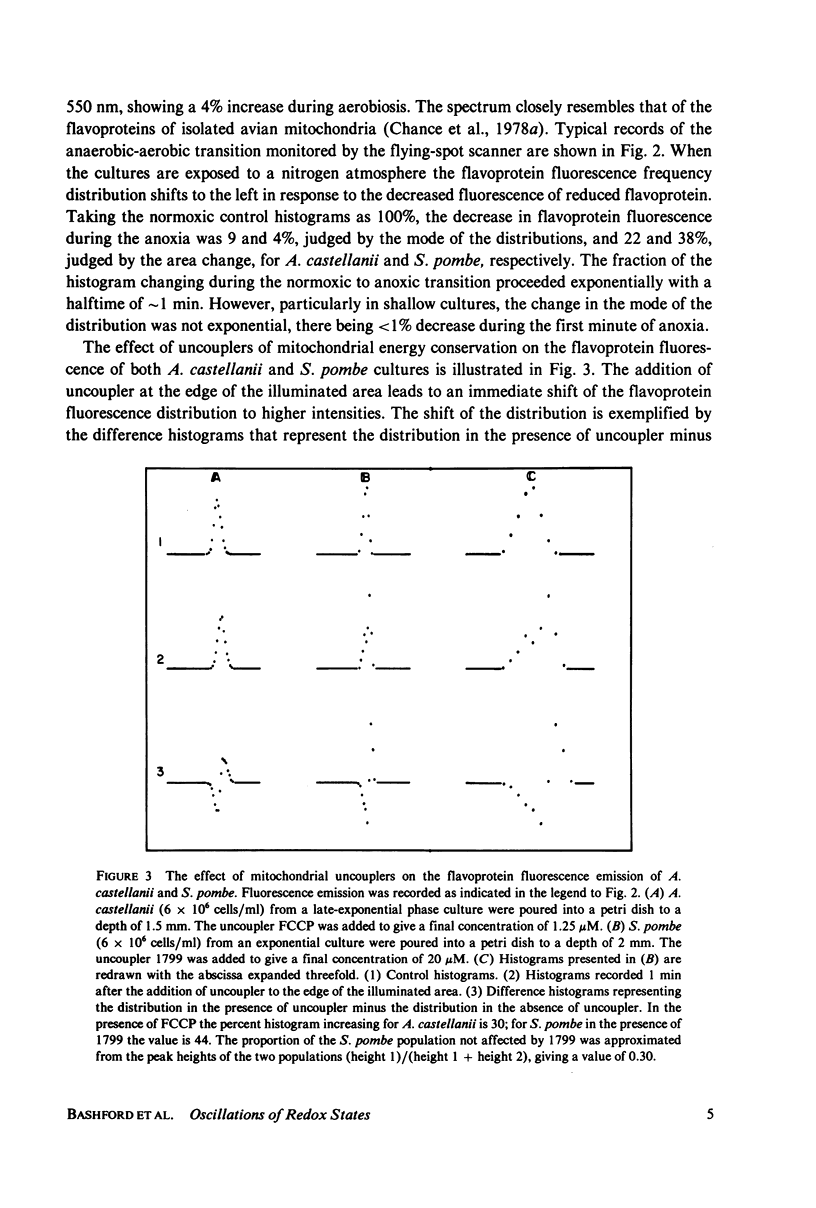
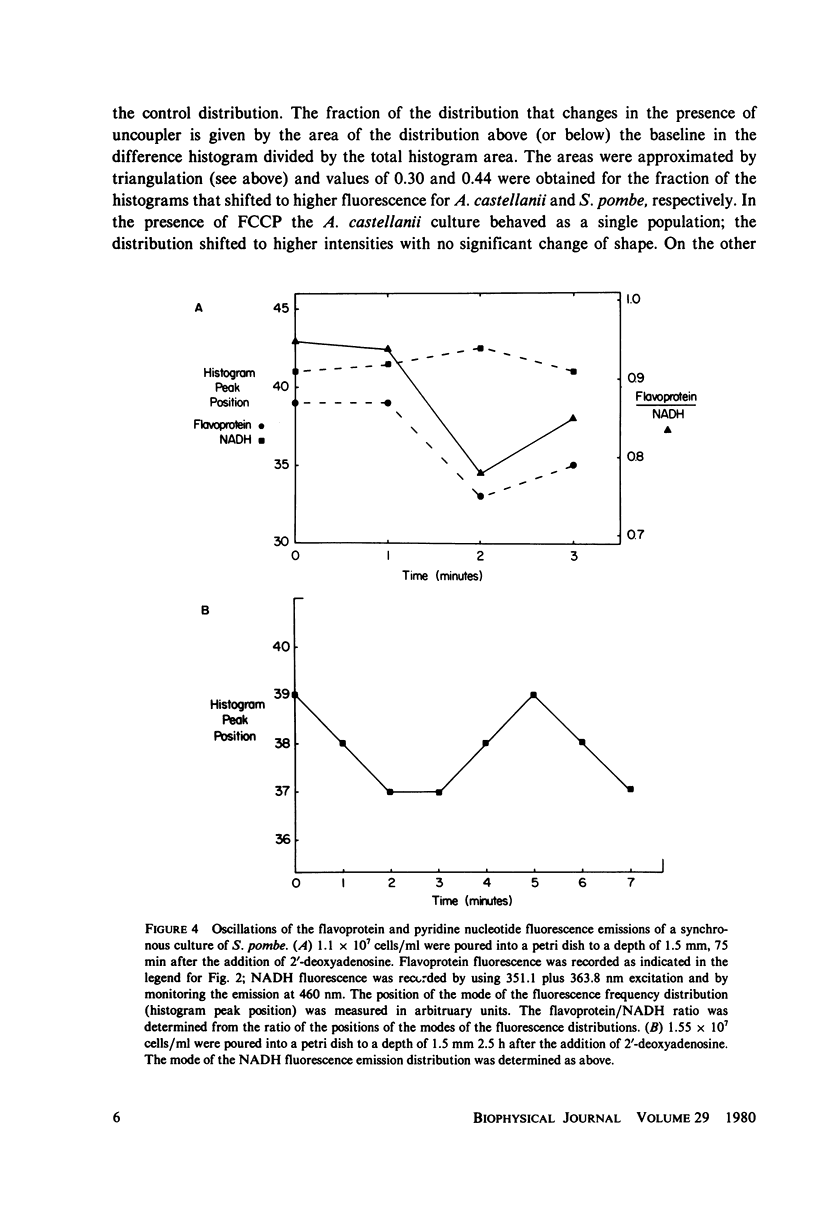
The redox state of the mitochondria of Acanthamoeba castellanii and Schizosaccharomyces pombe was assessed with a flying-spot fluorometer (Chance et al. 1978. Am. J. Physiol. 235:H 809) that provides excitation appropriate for oxidized flavoprotein or reduced pyridine nucleotide. Fluorescence signals could be resolved from the thin films of cultures that were only one cell deep. In both organisms anoxia was associated with an increased pyridine nucleotide and decreased flavoprotein fluorescence. The addition of mitochondrial uncoupling agents increased the flavoprotein fluorescence and the fluorometer was able to resolve uncoupler-sensitive and uncoupler-insensitive fractions of S. pombe cultures. In both synchronous and asynchronous cultures of A. castellanii and S. pombe the mitochondrial redox state oscillates with a period of 4.5 +/- 1.0 min. Oscillations with much longer period, of the order of an hour, are observed in synchronous cultures and these oscillations correlate with similar oscillations in respiratory rate, uncoupler sensitivity, and adenine nucleotide pool sizes. The results are consistent with the hypothesis that synchronous cultures of A. castellanii and S. pombe oscillate between the ADP-limited (state 4) and ADP-sufficient (state 3) respiratory states, i.e., exhibit in vivo respiratory control.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B. Phosphorylation efficiency of the intact cell. II. Crossover phenomena in bakers' yeast. J Biol Chem. 1959 Nov;234:3036–3040. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chance B., Barlow C., Nakase Y., Takeda H., Mayevsky A., Fischetti R., Graham N., Sorge J. Heterogeneity of oxygen delivery in normoxic and hypoxic states: a fluorometer study. Am J Physiol. 1978 Dec;235(6):H809–H820. doi: 10.1152/ajpheart.1978.235.6.H809. [DOI] [PubMed] [Google Scholar]
- Edwards C., Statham M., LLoyd D. The preparation of large-scale synchronous cultures of the trypanosomatid, Crithidia fasciculata,by cell-size selection: changes in respiration and adenylate charge through the cell-cycle. J Gen Microbiol. 1975 May;88(1):141–152. doi: 10.1099/00221287-88-1-141. [DOI] [PubMed] [Google Scholar]
- Edwards S. W., Chagla A. H., Griffiths A. J., Lloyd D. The cytochromes of Acanthamoeba castellanii. Biochem J. 1977 Oct 15;168(1):113–121. doi: 10.1042/bj1680113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison J. M., Creanor J. Induction synchrony in the fission yeast. Schizosaccharomyces pombe. Exp Cell Res. 1971 Aug;67(2):368–374. doi: 10.1016/0014-4827(71)90421-6. [DOI] [PubMed] [Google Scholar]



