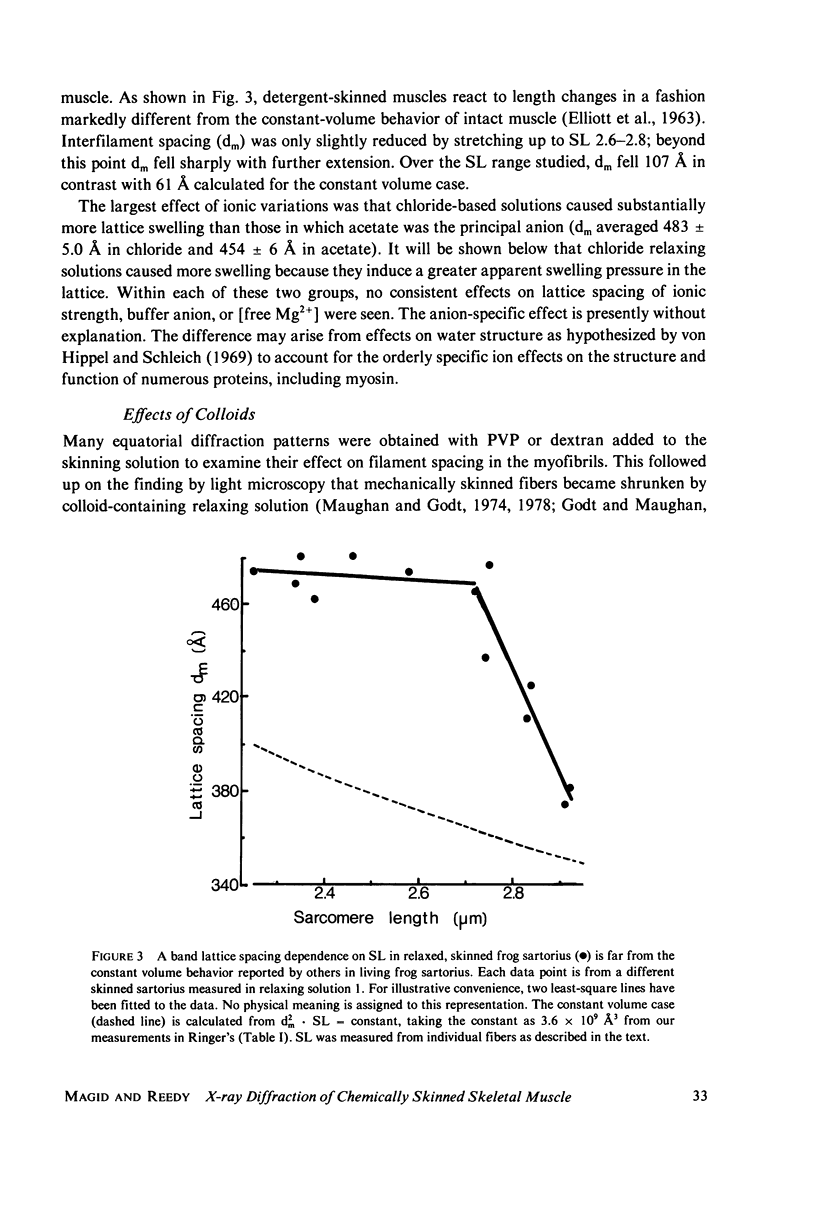
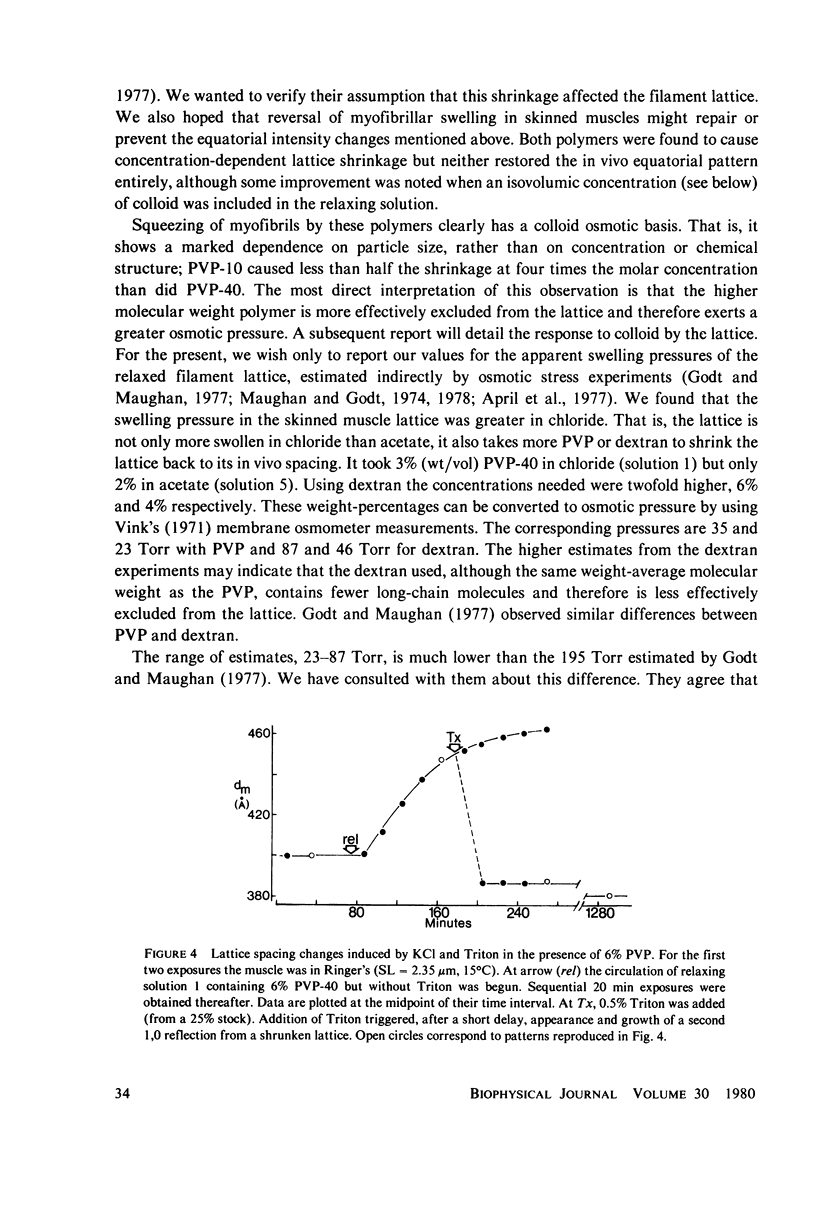
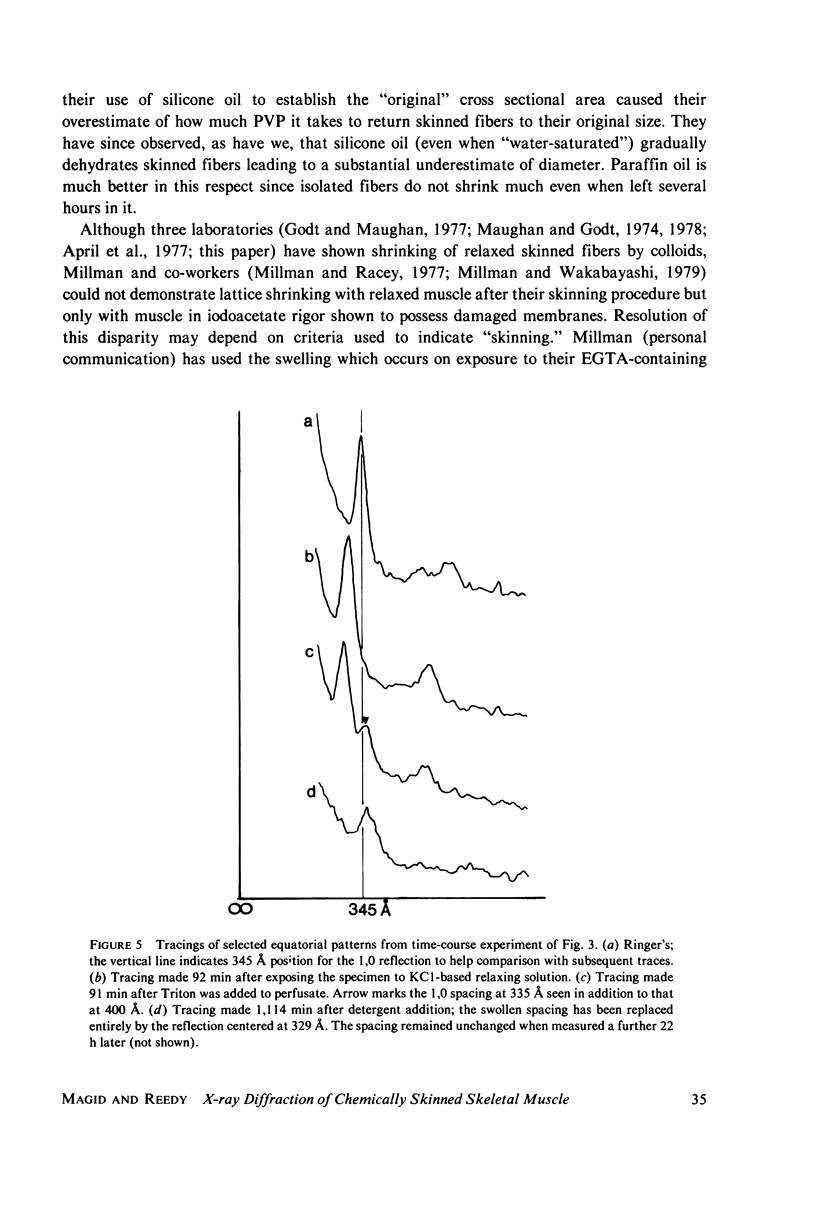
Abstract
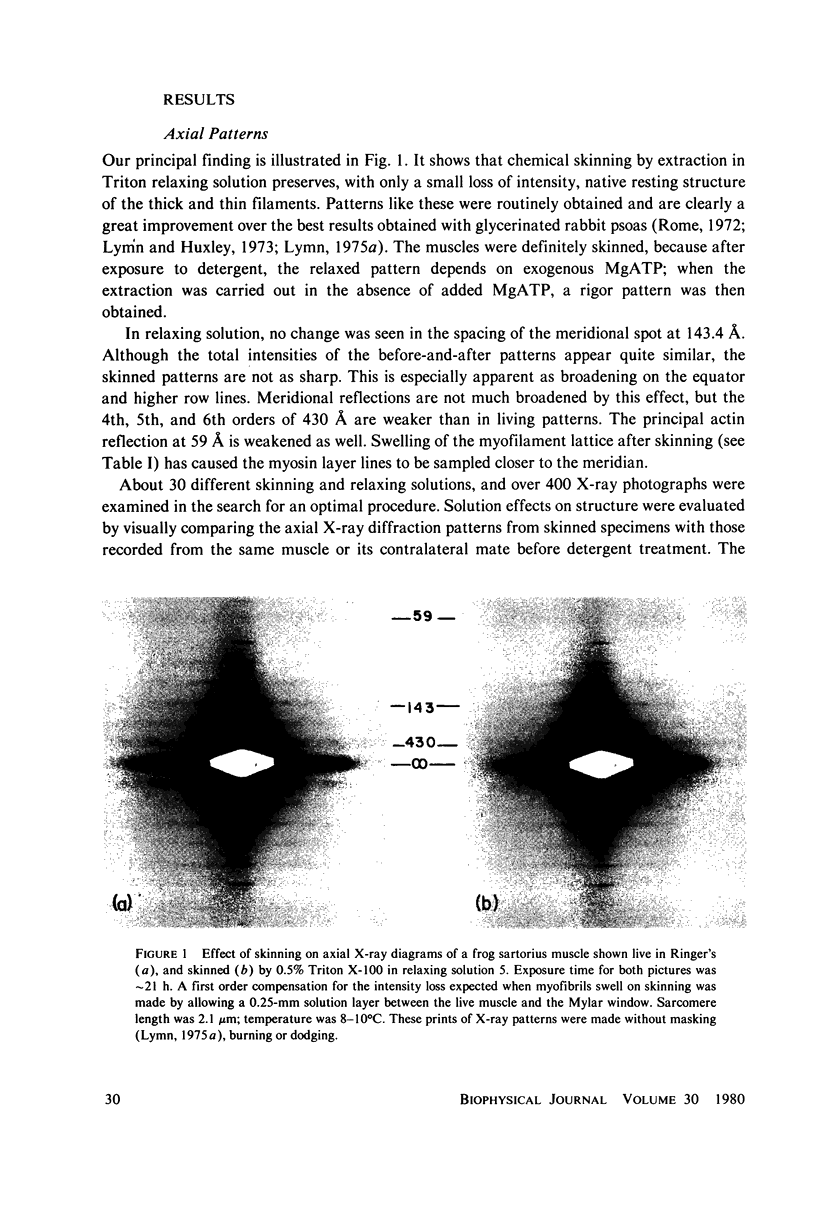
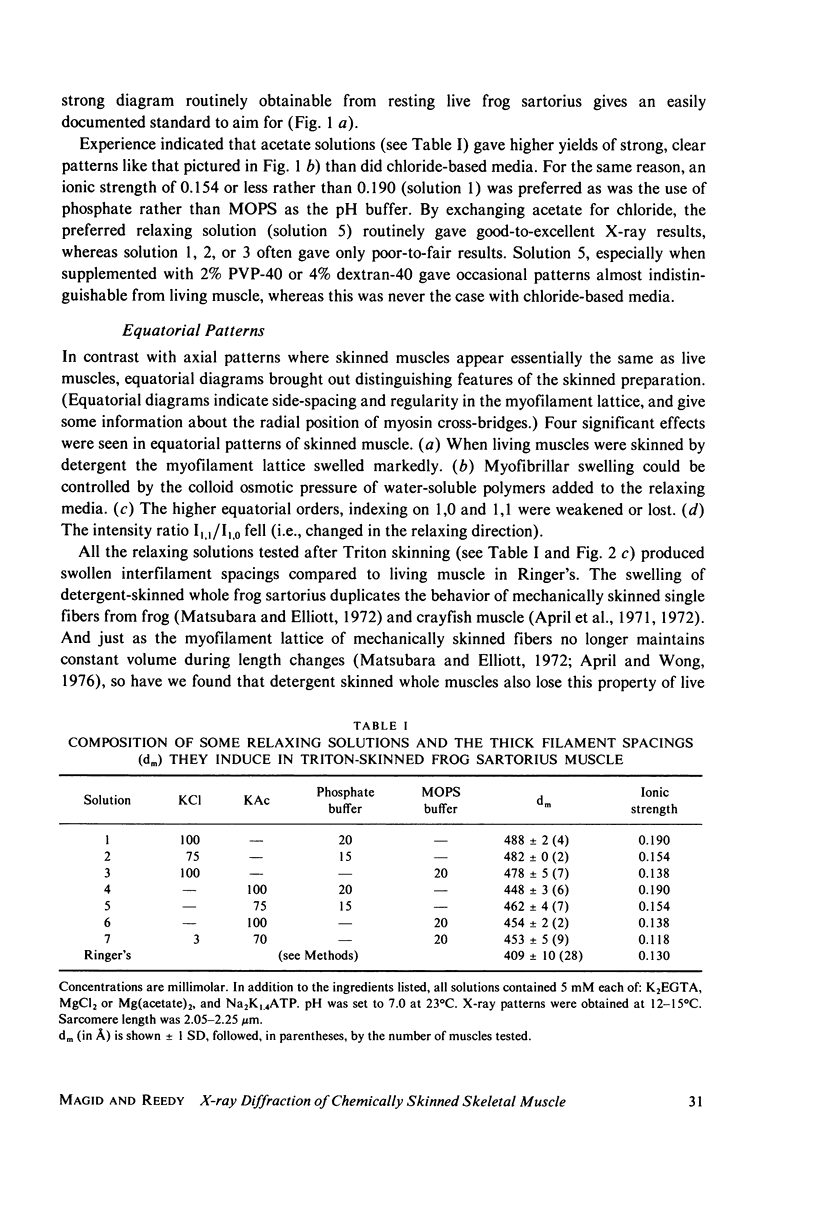
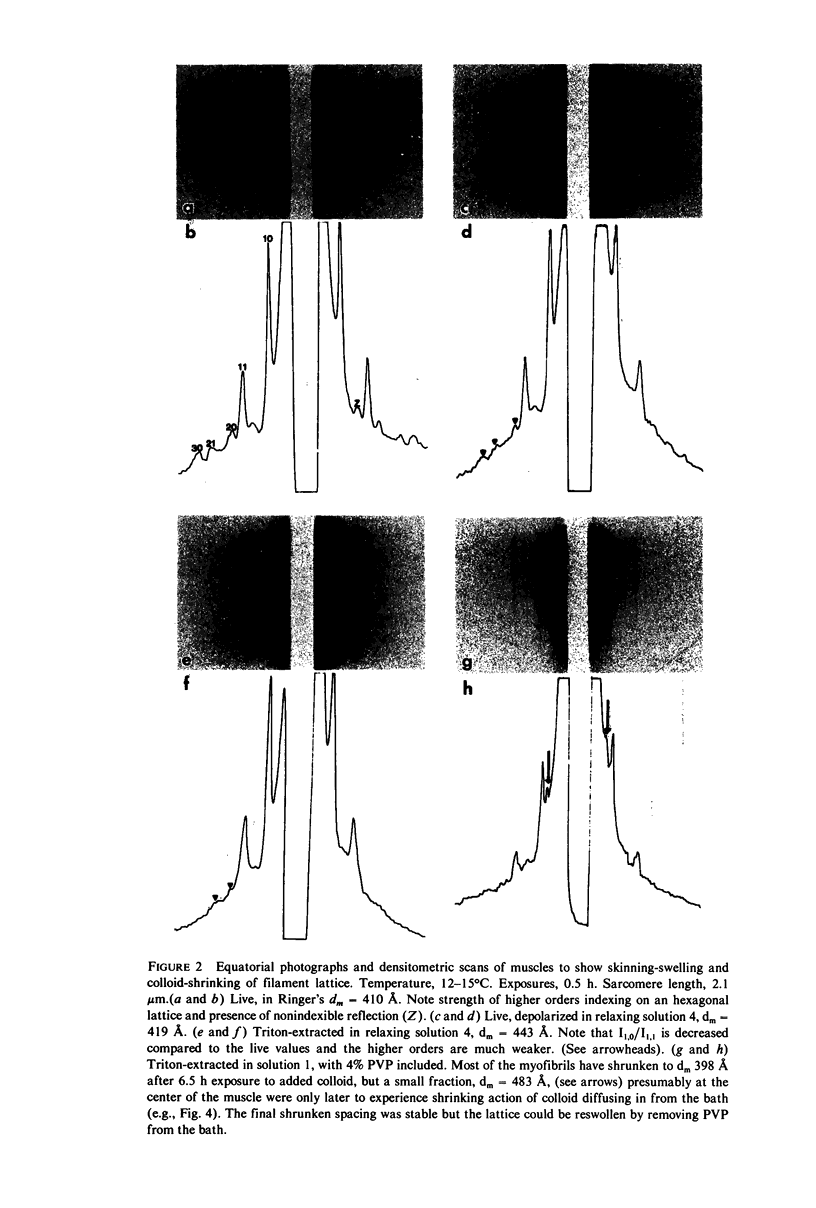
Whole frog sartorius muscles can be chemically skinned in approximately 2 h by relaxing solutions containing 0.5% Triton X-100. The intensity and order of the X-ray diffraction pattern from living muscle is largely retained after such skinning, indicating good retention of native structure in fibrils and filaments. Best X-ray results were obtained using a solution with (mM): 75 K acetate; 5 Mg acetate; 5 ATP; 5 EGTA; 15 K phosphate, 2% PVP, pH 7.0. Equatorial X-ray patterns showed that myofibrils swell after detergent skinning, as also observed after mechanical skinning. This swelling could be reversed by adding high molecular weight colloids (PVP or dextran) to the extracting solution. By finding the colloid osmotic pressure needed to restore the in vivo interfilament spacing (3% PVP, 4 X 10(4) mol wt) the swelling pressure was estimated as 35 Torr in a standard KCl-based relaxing solution. The swelling pressure and the extent of swelling were less than acetate replaced chloride as the major anion. Detergent-skinned muscle lost the constant-volume relation between sarcomere length and lattice spacing seen in intact muscle. Changes in A band spacing were paralleled by changes in I and band-Z line spacing at a constant sarcomere length. After detergent skinning, I1,0 rose while I1,1 fell, a change in the relaxing direction. Since raising the calcium ion concentrations from pCa 9 to PCa 6.7 was without effect on equatorial or axial X-ray patterns, we concluded that these intensity changes were not due to calcium-dependent cross-bridge movement but rather to disordering of thin filaments in the A band.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. H., Chaplain R. A. Preparation and properties of the contractile element of insect fibrillar muscle. J Cell Sci. 1966 Sep;1(3):311–330. doi: 10.1242/jcs.1.3.311. [DOI] [PubMed] [Google Scholar]
- April E. W., Brandt P. W., Elliott G. F. The myofilament lattice: studies on isolated fibers. I. The constancy of the unit-cell volume with variation in sarcomere length in a lattice in which the thin-to-thick myofilament ratio is 6:1. J Cell Biol. 1971 Oct;51(1):72–82. doi: 10.1083/jcb.51.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April E. W., Brandt P. W., Elliott G. F. The myofilament lattice: studies on isolated fibers. II. The effects of osmotic strength, ionic concentration, and pH upon the unit-cell volume. J Cell Biol. 1972 Apr;53(1):53–65. doi: 10.1083/jcb.53.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April E. W. Liquid-crystalline characteristics of the thick filament lattice of striated muscle. Nature. 1975 Sep 11;257(5522):139–141. doi: 10.1038/257139a0. [DOI] [PubMed] [Google Scholar]
- April E. W. The myofilament lattice: studies on isolated fibers. IV. Lattice equilibria in striated muscle. J Mechanochem Cell Motil. 1975;3(2):111–121. [PubMed] [Google Scholar]
- April E. W., Wong D. Non-isovolumic behavior of the unit cell of skinned striated muscle fibers. J Mol Biol. 1976 Feb 15;101(1):107–114. doi: 10.1016/0022-2836(76)90068-1. [DOI] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Elliott G. F., Lowy J., Millman B. M. Low-angle x-ray diffraction studies of living striated muscle during contraction. J Mol Biol. 1967 Apr 14;25(1):31–45. doi: 10.1016/0022-2836(67)90277-x. [DOI] [PubMed] [Google Scholar]
- Gibbons B. H., Gibbons I. R. Flagellar movement and adenosine triphosphatase activity in sea urchin sperm extracted with triton X-100. J Cell Biol. 1972 Jul;54(1):75–97. doi: 10.1083/jcb.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Maughan D. W. Swelling of skinned muscle fibers of the frog. Experimental observations. Biophys J. 1977 Aug;19(2):103–116. doi: 10.1016/S0006-3495(77)85573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. R. Contraction of detergent-treated smooth muscle. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3527–3530. doi: 10.1073/pnas.75.7.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Stewart M., Huxley H. E. Cross-bridge movement during muscle contraction. Nature. 1976 Jun 17;261(5561):606–608. doi: 10.1038/261606a0. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J Mol Biol. 1975 Feb 15;92(1):113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial x-ray diagram. J Mol Biol. 1968 Nov 14;37(3):507–520. doi: 10.1016/0022-2836(68)90118-6. [DOI] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W. Equatorial X-ray reflections and cross arm movement in skeletal muscle. Nature. 1975 Dec 25;258(5537):770–772. doi: 10.1038/258770a0. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. Low-angle x-ray diagrams from skeletal muscle: the effect of AMP-PNP, a non-hydrolyzed analogue of ATP. J Mol Biol. 1975 Dec 25;99(4):567–582. doi: 10.1016/s0022-2836(75)80172-0. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Elliott G. F. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol. 1972 Dec 30;72(3):657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Sollins M. R., Julian F. J. Calcium activation produces a characteristic response to stretch in both skeletal and cardiac muscle. Nature. 1976 Apr 15;260(5552):619–621. doi: 10.1038/260619a0. [DOI] [PubMed] [Google Scholar]
- Orentlicher M., Reuben J. P., Grundfest H., Brandt P. W. Calcium binding and tension development in detergent-treated muscle fibers. J Gen Physiol. 1974 Feb;63(2):168–186. doi: 10.1085/jgp.63.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky R. J., St Onge H., Yu L., Lymn R. W. X-ray diffraction of actively shortening muscle. Proc Natl Acad Sci U S A. 1976 Mar;73(3):813–817. doi: 10.1073/pnas.73.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome E. Relaxation of glycerinated muscle: low-angle x-ray diffraction studies. J Mol Biol. 1972 Mar 28;65(2):331–345. doi: 10.1016/0022-2836(72)90285-9. [DOI] [PubMed] [Google Scholar]
- Small J. V., Celis J. E. Filament arrangements in negatively stained cultured cells: the organization of actin. Cytobiologie. 1978 Feb;16(2):308–325. [PubMed] [Google Scholar]
- Small J. V. Studies on isolated smooth muscle cells: The contractile apparatus. J Cell Sci. 1977 Apr;24:327–349. doi: 10.1242/jcs.24.1.327. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Pang D. C., Briggs F. N. The purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta. 1971 Aug 6;245(1):259–262. doi: 10.1016/0005-2728(71)90033-8. [DOI] [PubMed] [Google Scholar]
- Toylor D. L. Quantitative studies on the polarization optical properties of striated muscle. I. Birefringence changes of rabbit psoas muscle in the transition from rigor to relaxed state. J Cell Biol. 1976 Mar;68(3):497–511. doi: 10.1083/jcb.68.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. S., Zollman J., Reuben J. P., Brandt P. W. Human skeletal muscle: properties of the "chemically skinned%" fiber. Science. 1975 Mar 21;187(4181):1075–1076. doi: 10.1126/science.187.4181.1075. [DOI] [PubMed] [Google Scholar]
- Wray J., Vibert P., Cohen C. Actin filaments in muscle: pattern of myosin and tropomyosin/troponin attachments. J Mol Biol. 1978 Sep 25;124(3):501–521. doi: 10.1016/0022-2836(78)90184-5. [DOI] [PubMed] [Google Scholar]
- Yu L. C., Lymn R. W., Podolsky R. J. Characterization of a non-indexible equatorial x-ray reflection from frog sartorius muscle. J Mol Biol. 1977 Sep 25;115(3):455–464. doi: 10.1016/0022-2836(77)90165-6. [DOI] [PubMed] [Google Scholar]