Abstract
The space-clamped squid axon membrane and two versions of the Hodgkin-Huxley model (the original, and a strongly adapting version) are subjected to a first order dynamic analysis. Stable, repetitive firing is induced by phase-locking nerve impulses to sinusoidal currents. The entrained impulses are then pulse position modulated by additional, small amplitude perturbation sinusoidal currents with respect to which the frequencies response of impulse density functions are measured. (Impulse density is defined as the number of impulses per unit time of an ensemble of membranes with each membrane subject to the same stimulus). Two categories of dynamic response are observed: one shows clear indications of a corner frequency, the other has the corner frequency obscured by dynamics associated with first order conductance perturbations in the interspike interval. The axon membrane responds with first order perturbations whereas the unmodified Hodgkin-Huxley model does not. Quantitative dynamic signatures suggest that the relaxation times of axonal recovery excitation variables are twice as long as those of the corresponding model variables. A number of other quantitative differences between axon and models, including the values of threshold stimuli are also observed.
Full text
PDF

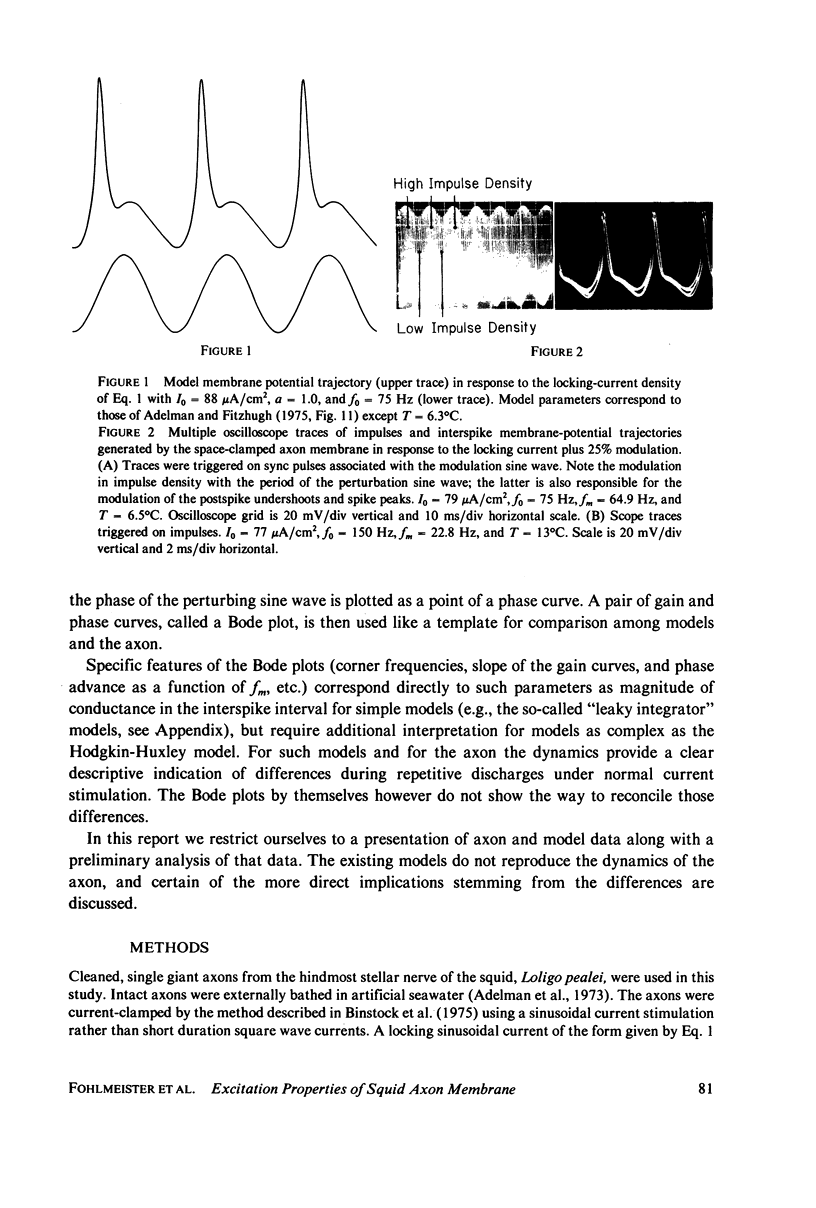




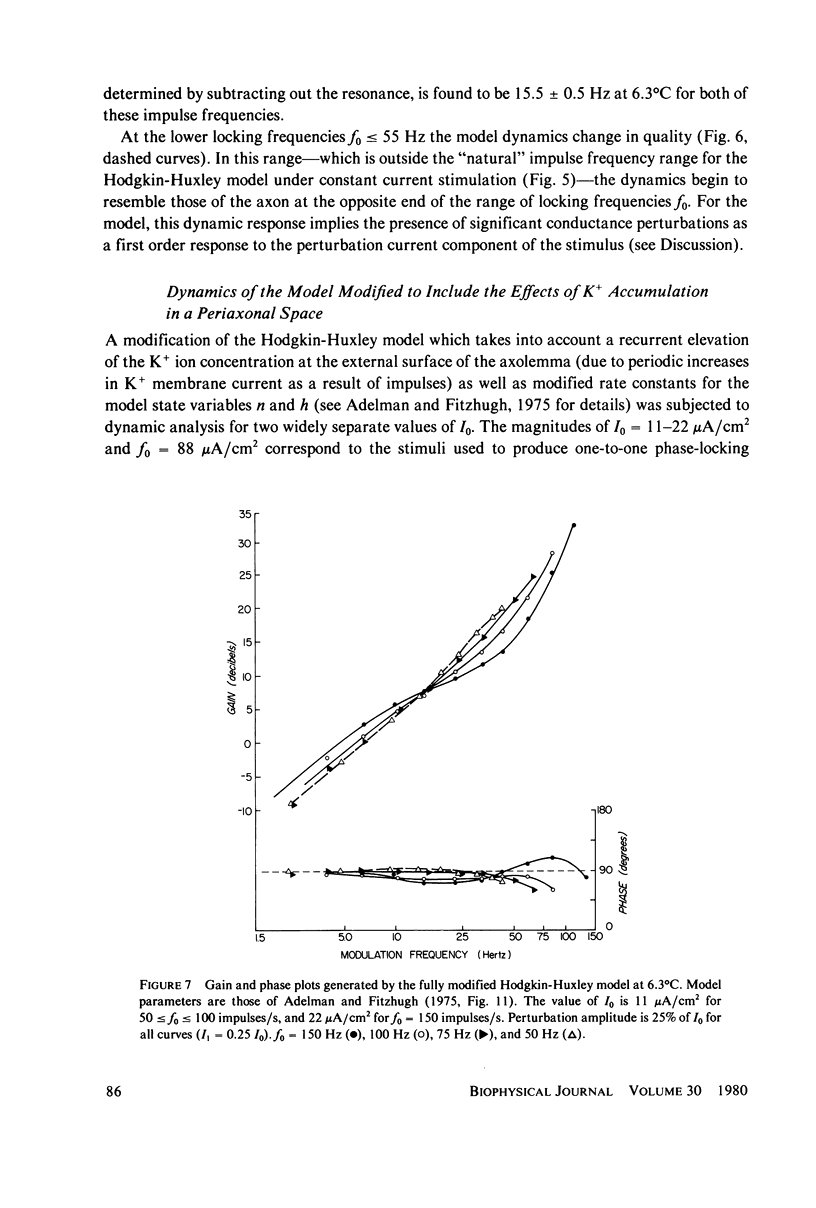











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman W. J., Jr, Fitzhugh R. Solutions of the Hodgkin-Huxley equations modified for potassium accumulation in a periaxonal space. Fed Proc. 1975 Apr;34(5):1322–1329. [PubMed] [Google Scholar]
- Adelman W. J., Jr, Palti Y., Senft J. P. Potassium ion accumulation in a periaxonal space and its effect on the measurement of membrane potassium ion conductance. J Membr Biol. 1973 Nov 8;13(4):387–410. doi: 10.1007/BF01868237. [DOI] [PubMed] [Google Scholar]
- Best E. N. Null space in the Hodgkin-Huxley Equations. A critical test. Biophys J. 1979 Jul;27(1):87–104. doi: 10.1016/S0006-3495(79)85204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock L., Adelman W. J., Jr, Senft P., Lecar H. Determination of the resistance in series with the membranes of giant axons. J Membr Biol. 1975 Apr 23;21(1-2):25–47. doi: 10.1007/BF01941060. [DOI] [PubMed] [Google Scholar]
- Fohlmeister J. F. Adaptation and accommodation in the squid axon. Biol Cybern. 1975;18(1):49–60. doi: 10.1007/BF00337055. [DOI] [PubMed] [Google Scholar]
- Fohlmeister J. F., Poppele R. E., Purple R. L. Repetitive firing: a quantitative study of feedback in model encoders. J Gen Physiol. 1977 Jun;69(6):815–848. doi: 10.1085/jgp.69.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohlmeister J. F., Poppele R. E., Purple R. L. Repetitive firing: dynamic behavior of sensory neurons reconciled with a quantitative model. J Neurophysiol. 1974 Nov;37(6):1213–1227. doi: 10.1152/jn.1974.37.6.1213. [DOI] [PubMed] [Google Scholar]
- Fohlmeister J. F., Poppele R. E., Purple R. L. Repetitive firing: quantitative analysis of encoder behavior of slowly adapting stretch receptor of crayfish and eccentric cell of Limulus. J Gen Physiol. 1977 Jun;69(6):849–877. doi: 10.1085/jgp.69.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohlmeister J. A theoretical study of neural adaptation and transient responses due to inhibitory feedback. Bull Math Biol. 1979;41(3):257–282. doi: 10.1007/BF02460812. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., OOMURA Y. The critical depolarization for the spike in the squid giant axon. Jpn J Physiol. 1958 Sep 15;8(3):234–245. doi: 10.2170/jjphysiol.8.234. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden A. V. The response of excitable membrane models to a cyclic input. Biol Cybern. 1976 Jan 2;21(1):1–7. doi: 10.1007/BF00326666. [DOI] [PubMed] [Google Scholar]
- Jakobsson E. A fully coupled transient excited state model for the sodium channel. II. Implications for action potential generation, threshold, repetitive firing, and accommodation. J Math Biol. 1978 Aug 31;6(3):235–248. doi: 10.1007/BF02547799. [DOI] [PubMed] [Google Scholar]
- Poppele R. E., Chen W. J. Repetitive firing behavior of mammalian muscle spindle. J Neurophysiol. 1972 May;35(3):357–364. doi: 10.1152/jn.1972.35.3.357. [DOI] [PubMed] [Google Scholar]
- Rescigno A., Stein R. B., Purple R. L., Poppele R. E. A neuronal model for the discharge patterns produced by cyclic inputs. Bull Math Biophys. 1970 Sep;32(3):337–353. doi: 10.1007/BF02476873. [DOI] [PubMed] [Google Scholar]




