Abstract
The theory of fluorescent emission anisotropy [r(t)] of a cylindrical probe in a membrane suspension is developed. It is shown, independent of any model, that the limiting anisotropy [r(infinity)] is proportional to the square to the order parameter of the probe. The order parameter determines the first nontrivial term in the expansion of the equilibrium orientational distribution function of the probe in a series of Legendre polynomials. Following Kinosita, Kawato, and Ikegami, the motion of the probe is described as diffusion ("wobbling") within a cone of semiangle theta 0. Within the framework of this model, an accurate single-exponential approximation for r(t) is considered. An analytic expression relating the effective relaxation time, which appears in the above approximation, to theta 0 and the diffusion coefficient for wobbling is derived. The model is generalized to the situation where the probe is attached to a macromolecule whose motion cannot be neglected on the time scale of the fluorescence experiment. Finally, by exploiting the formal similarity between the theory of fluorescence depolarization and 13C-NMR dipolar relaxation, expressions for T1, T2, and the nuclear Overhauser enhancement are derived for a protonated carbon which is nonrigidly attached to a macromolecule and undergoes librational motion described as diffusion on a spherical "cap" of semiangle theta 0.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kawato S., Kinosita K., Jr, Ikegami A. Effect of cholesterol on the molecular motion in the hydrocarbon region of lecithin bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1978 Nov 14;17(23):5026–5031. doi: 10.1021/bi00616a026. [DOI] [PubMed] [Google Scholar]
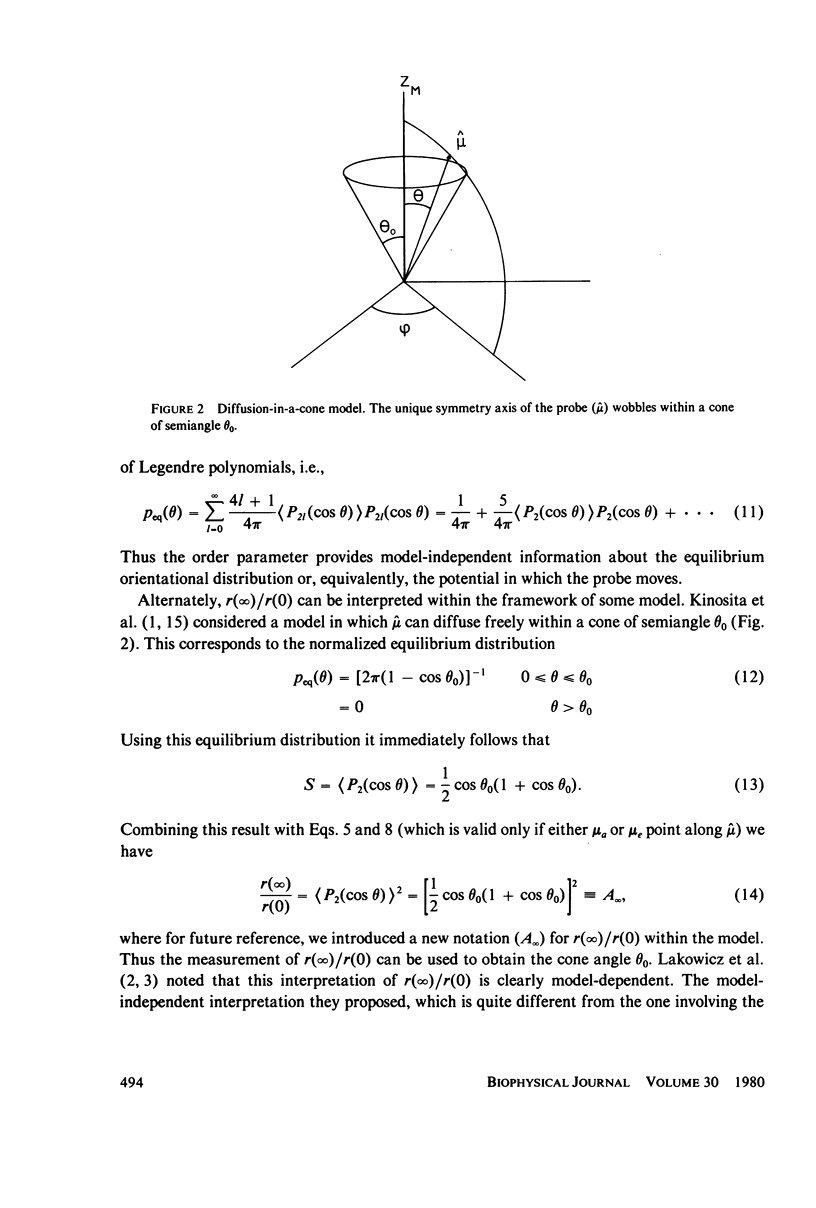
- Kinosita K., Jr, Kawato S., Ikegami A. A theory of fluorescence polarization decay in membranes. Biophys J. 1977 Dec;20(3):289–305. doi: 10.1016/S0006-3495(77)85550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G., Hogen D. Differential polarized phase fluorometric investigations of diphenylhexatriene in lipid bilayers. Quantitation of hindered depolarizing rotations. Biochemistry. 1979 Feb 6;18(3):508–519. doi: 10.1021/bi00570a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G. Quantitation of hindered rotations of diphenylhexatriene in lipid bilayers by differential polarized phase fluorometry. Science. 1978 Jun 23;200(4348):1399–1401. doi: 10.1126/science.663620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro I., Pecht I., Stryer L. Subnanosecond motions of tryptophan residues in proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):56–60. doi: 10.1073/pnas.76.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]



