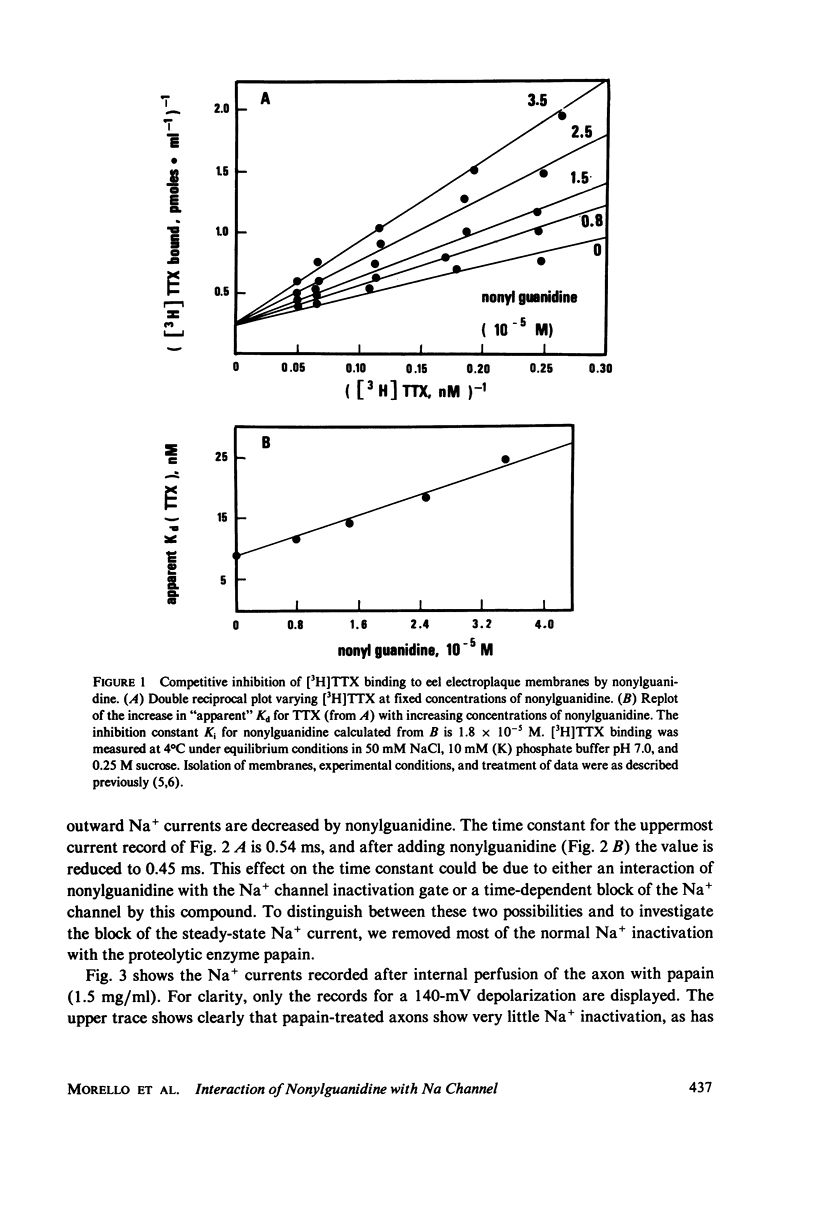
Abstract
Alkyl and aromatic guanidines interact strongly with the tetrodotoxin (TTX)- receptor site in eel electroplaque membranes, showing competition with TTX. That these guanidines could be useful as highly reversible small molecular weight blockers of Na+ currents is therefore suggested. We have investigated the mechanisms of interaction of one of these derivatives, nonylguanidine, by studying its effects on Na+ currents in squid giant axons using voltage clamp techniques. Although nonylguanidine competed with TTX for binding to eel electroplaque membrane fragments (Ki = 1.8 X 10(-5) M), it reversibly blocked both inward and outward Na+ currents in intact axons only if applied to the interior. In axons with the Na+ inactivation removed by papain nonylguanidine produced a time-dependent block very similar to that reported for strychnine and pancuronium. The reduction of steady-state currents in these axons was also voltage-dependent, with increasing block observed with increasing step depolarization. These results suggest that nonylguanidine binds to a site accessible from the axoplasmic side of the channel, simulating Na+ inactivation in papain-treated axons and competing with the normal inactivation process in untreated axons. The competition between internal nonylguanidine and external TTX may result from perturbation by the positively charged nonylguanidine of the TTX-binding site from within the channel itself.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Rubinson K. A. Chemical modification of crab nerves can make them insensitive to the local anaesthetics tetrodotoxin and saxitoxin. Nature. 1975 Oct 2;257(5525):412–414. doi: 10.1038/257412a0. [DOI] [PubMed] [Google Scholar]
- Begenisich T., Lynch C. Effects of internal divalent cations on voltage-clamped squid axons. J Gen Physiol. 1974 Jun;63(6):675–689. doi: 10.1085/jgp.63.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. C., Brodwick M. S., Oxford G. S., Rudy B. Arginine-specific reagents remove sodium channel inactivation. Nature. 1978 Feb 2;271(5644):473–476. doi: 10.1038/271473a0. [DOI] [PubMed] [Google Scholar]
- Hille B. The receptor for tetrodotoxin and saxitoxin. A structural hypothesis. Biophys J. 1975 Jun;15(6):615–619. doi: 10.1016/S0006-3495(75)85842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Anderson N. C., Moore J. W. Tetrodotoxin does not block excitation from inside the nerve membrane. Science. 1966 Aug 12;153(3737):765–767. doi: 10.1126/science.153.3737.765. [DOI] [PubMed] [Google Scholar]
- Reed J. K., Raftery M. A. Properties of the tetrodotoxin binding component in plasma membranes isolated from Electrophorus electricus. Biochemistry. 1976 Mar 9;15(5):944–953. doi: 10.1021/bi00650a002. [DOI] [PubMed] [Google Scholar]
- Reed J. K., Trzos W. Interaction of substituted guanidines with the tetrodotoxin-binding component in Electrophorus electricus. Arch Biochem Biophys. 1979 Jul;195(2):414–422. doi: 10.1016/0003-9861(79)90368-0. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Rojas E., Rudy B. Destruction of the sodium conductance inactivation by a specific protease in perfused nerve fibres from Loligo. J Physiol. 1976 Nov;262(2):501–531. doi: 10.1113/jphysiol.1976.sp011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager P., Profera C. Inhibition of the receptor for tetrodotoxin in nerve membranes by reagents modifying carboxyl groups. Biochim Biophys Acta. 1973 Aug 9;318(1):141–146. doi: 10.1016/0005-2736(73)90343-x. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Narahashi T. Kinetic analysis of pancuronium interaction with sodium channels in squid axon membranes. J Gen Physiol. 1977 Mar;69(3):293–323. doi: 10.1085/jgp.69.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]



