Abstract
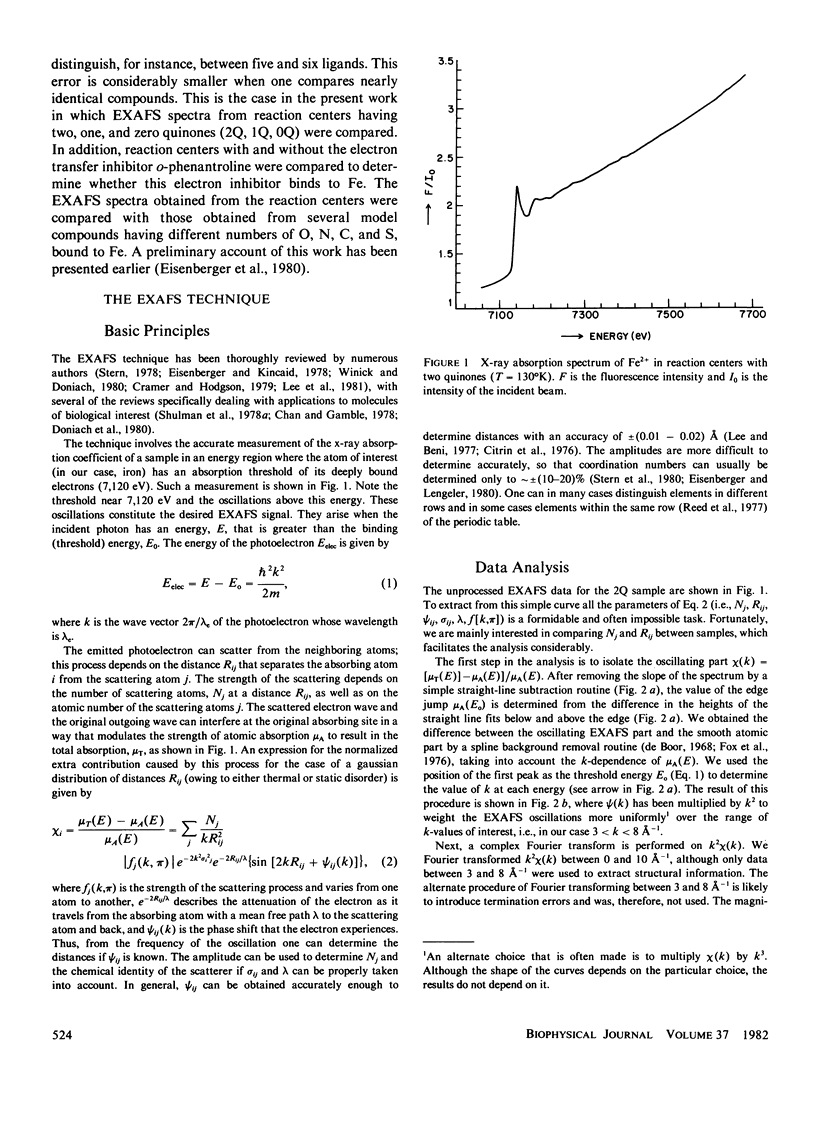
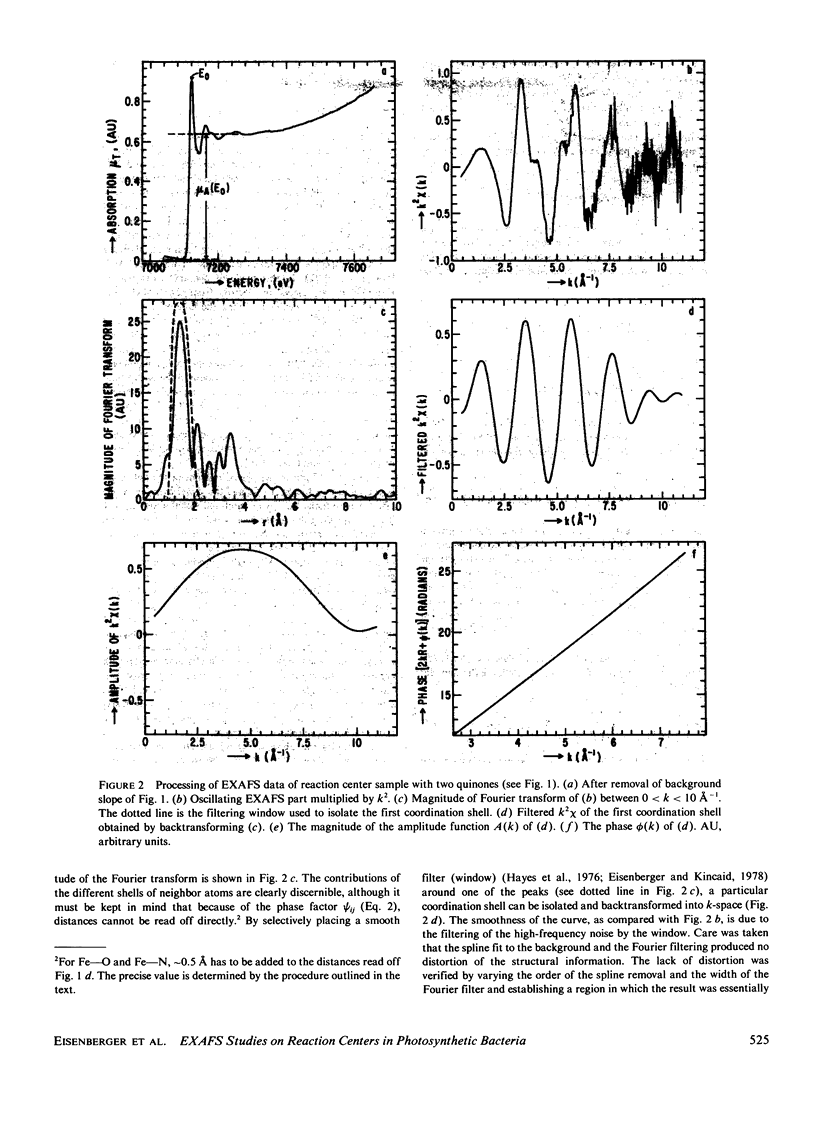
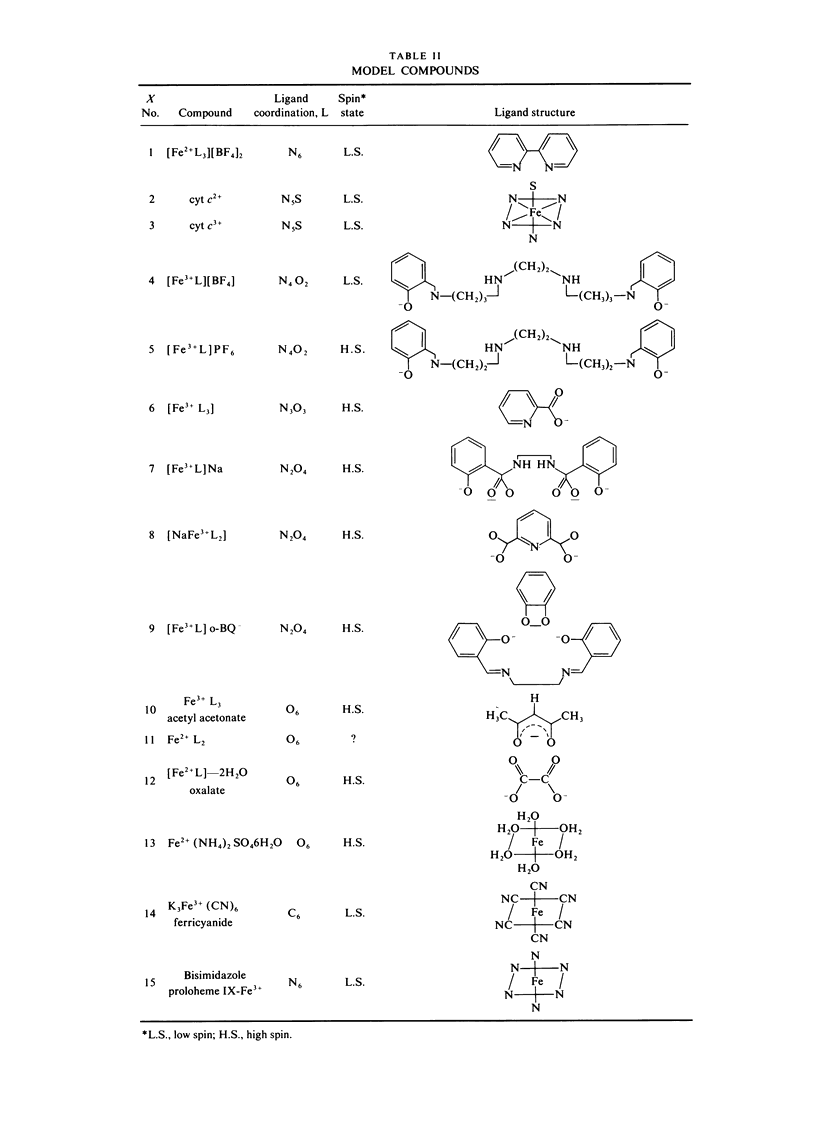
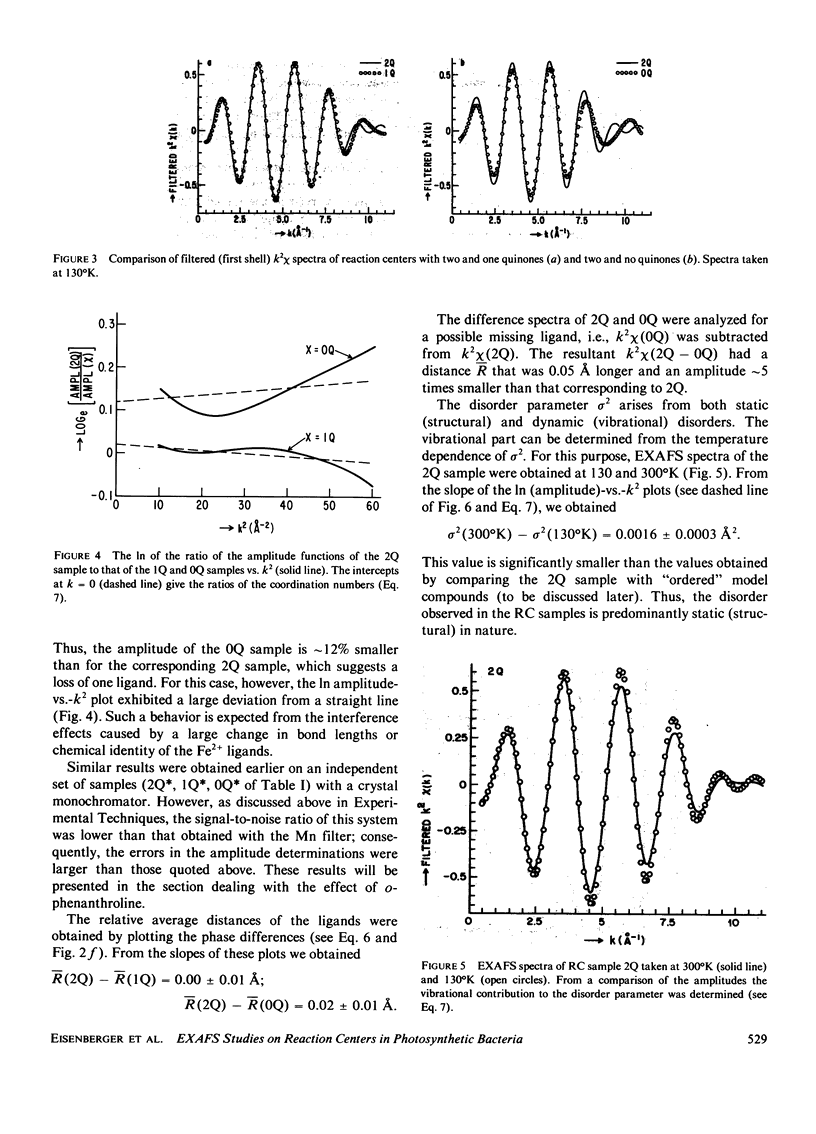
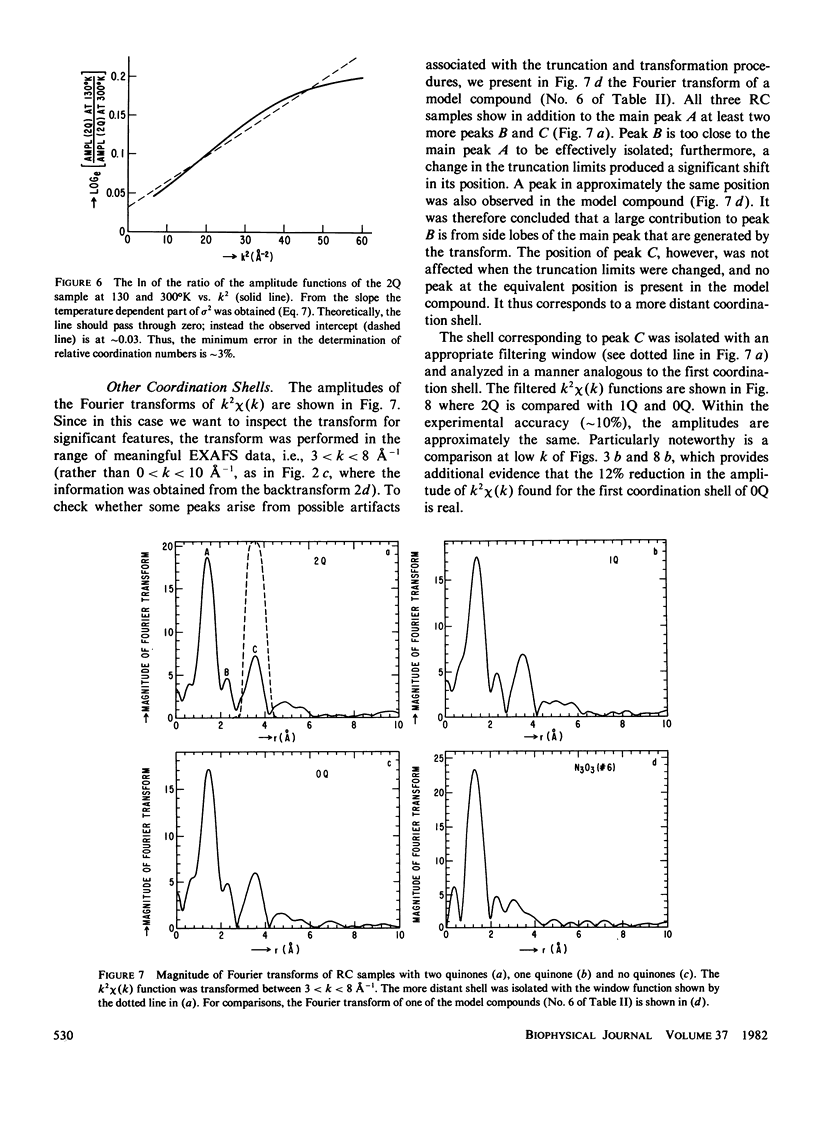
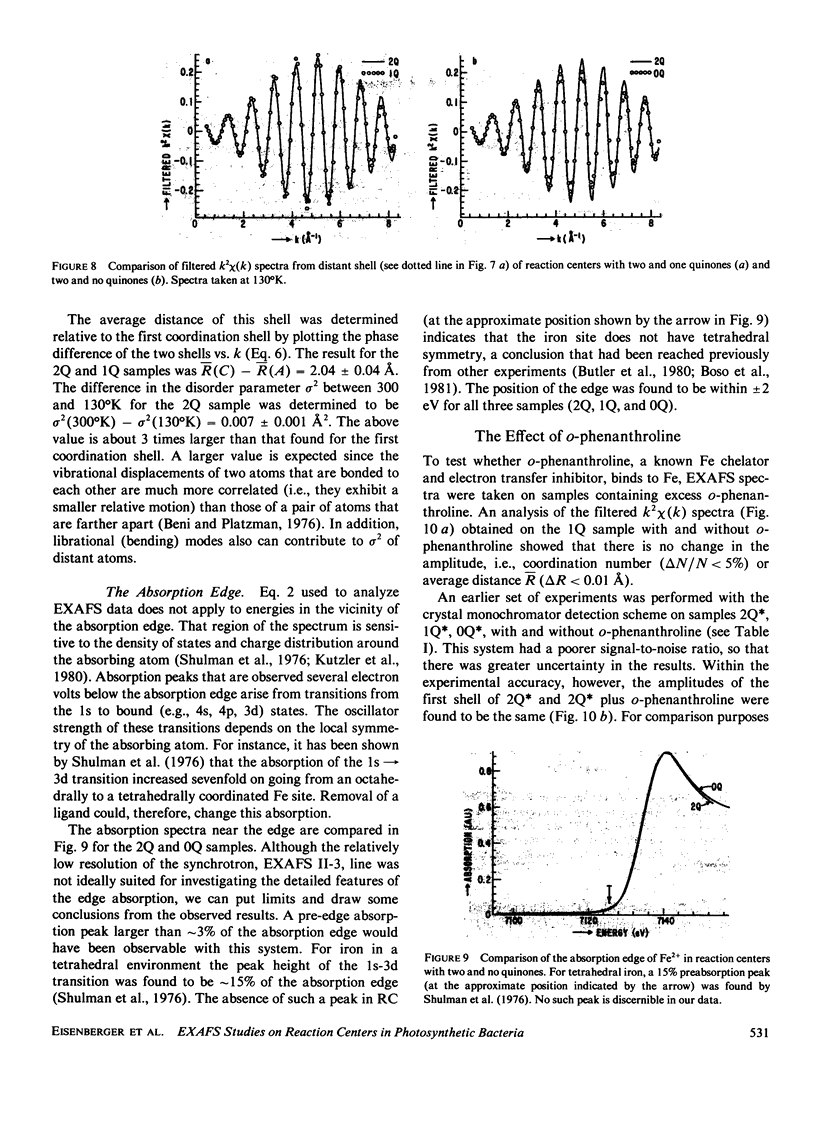
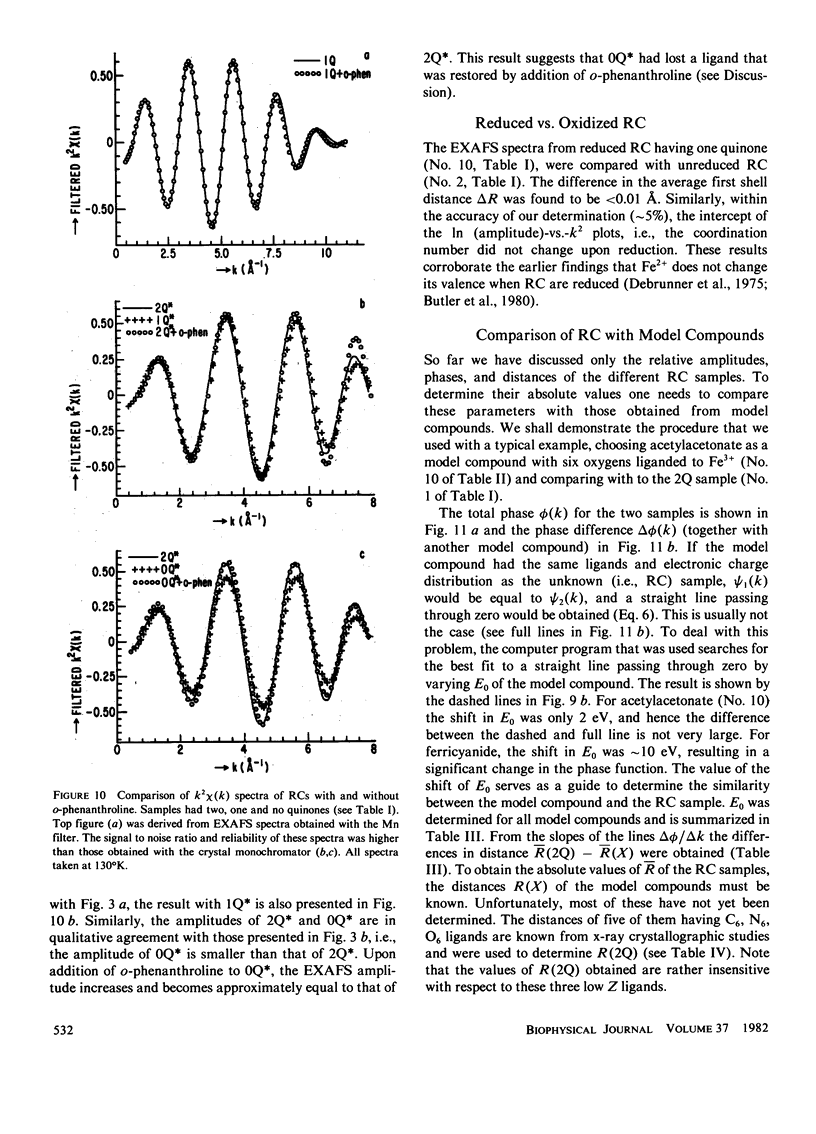
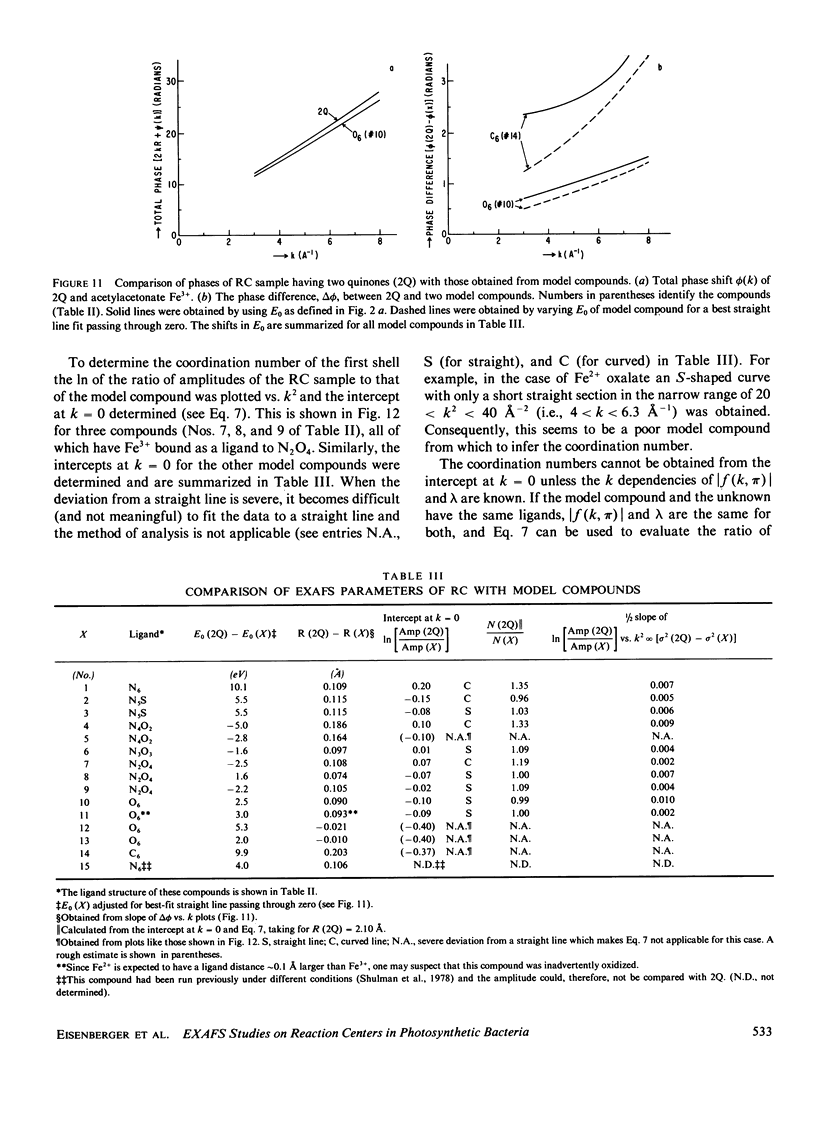
Extended x-ray absorption fine structure (EXAFS) studies were performed on reaction centers (RC) of the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26. RC containing two, one, and no quinones (2Q, 1Q, 0Q) samples were studied. The average ligand distance of the first coordination shell was determined to be 2.10 +/- 0.02 A with a more distant shell at 4.14 +/- 0.05 A. The Fe2+ site in RC was found to have a very large structural disorder parameter, from which a spread in ligand distance per iron site of approximately +/- 0.1 A was deduced. The most likely coordination number of the first shell is six, with a mixture of oxygens and nitrogens as ligands. The edge absorption results are consistent with the Fe2+ being in distorted octahedral environment. The EXAFS spectra of the 2Q and 1Q samples with and without O-phenanthroline were found to be the same. This indicates that either the secondary quinone and o-phenanthroline do not bind to Fe2+ or that they replace an equivalent ligand. The 0Q sample showed a 12% decrease in the EXAFS amplitude, which was restored upon addition of o-phenanthroline. These results can be explained by either a loss of a ligand or a severe conformational change when the primary quinone was removed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship R. E., Parson W. W. The involvement of iron and ubiquinone in electron transfer reactions mediated by reaction centers from photosynthetic bacteria. Biochim Biophys Acta. 1979 Mar 15;545(3):429–444. doi: 10.1016/0005-2728(79)90152-x. [DOI] [PubMed] [Google Scholar]
- Bunker G., Stern E. A., Blankenship R. E., Parson W. W. An x-ray absorption study of the iron site in bacterial photosynthetic reaction centers. Biophys J. 1982 Feb;37(2):539–551. doi: 10.1016/S0006-3495(82)84699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. F., Johnston D. C., Shore H. B., Fredkin D. R., Okamura M. Y., Feher G. The electronic structure of Fe2+ in reaction centers from Rhodopseudomonas sphaeroides. I. Static magnetization measurements. Biophys J. 1980 Dec;32(3):967–992. doi: 10.1016/S0006-3495(80)85030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. I., Gamble R. C. X-ray absorption spectroscopy of metalloproteins. Methods Enzymol. 1978;54:323–345. doi: 10.1016/s0076-6879(78)54022-6. [DOI] [PubMed] [Google Scholar]
- Collins D. M., Countryman R., Hoard J. L. Stereochemistry of low-spin iron porphyrins. I. Bis(imidazole)- , , , -tetraphenylporphinatoiron(3) chloride. J Am Chem Soc. 1972 Mar 22;94(6):2066–2072. doi: 10.1021/ja00761a045. [DOI] [PubMed] [Google Scholar]
- Eisenberger P., Kincaid B. M. EXAFS: new horizons in structure determinations. Science. 1978 Jun 30;200(4349):1441–1447. doi: 10.1126/science.663627. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D., Okamura M. Y., Feher G. Interaction of cytochrome c with reaction centers of Rhodopseudomonas sphaeroides R-26: determination of number of binding sites and dissociation constants by equilibrium dialysis. Biochemistry. 1980 Dec 9;19(25):5687–5692. doi: 10.1021/bi00566a004. [DOI] [PubMed] [Google Scholar]
- Shulman G. R., Yafet Y., Eisenberger P., Blumberg W. E. Observations and interpretation of x-ray absorption edges in iron compounds and proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1384–1388. doi: 10.1073/pnas.73.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R. G., Eisenberger P., Kincaid B. M. X-ray absorption spectroscopy of biological molecules. Annu Rev Biophys Bioeng. 1978;7:559–578. doi: 10.1146/annurev.bb.07.060178.003015. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Eisenberger P., Teo B. K., Kincaid B. M., Brown G. S. Fluorescence X-ray adsorption studies of rubredoxin and its model compounds. J Mol Biol. 1978 Sep 15;124(2):305–321. doi: 10.1016/0022-2836(78)90301-7. [DOI] [PubMed] [Google Scholar]
- Willoughby J. O., Menadue M., Zeegers P., Wise P. H., Oliver J. R. Effects of human growth hormone on the secretion of rat growth hormone. J Endocrinol. 1980 Jul;86(1):165–169. doi: 10.1677/joe.0.0860165. [DOI] [PubMed] [Google Scholar]



