Abstract
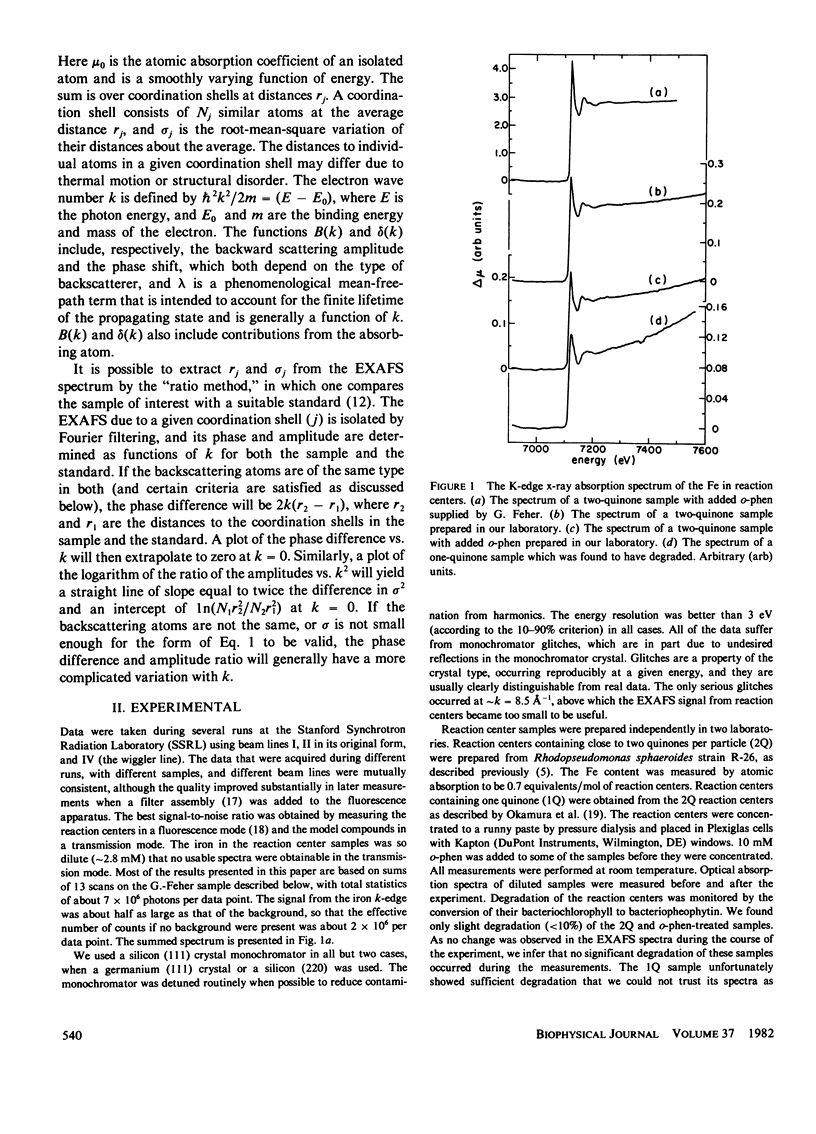
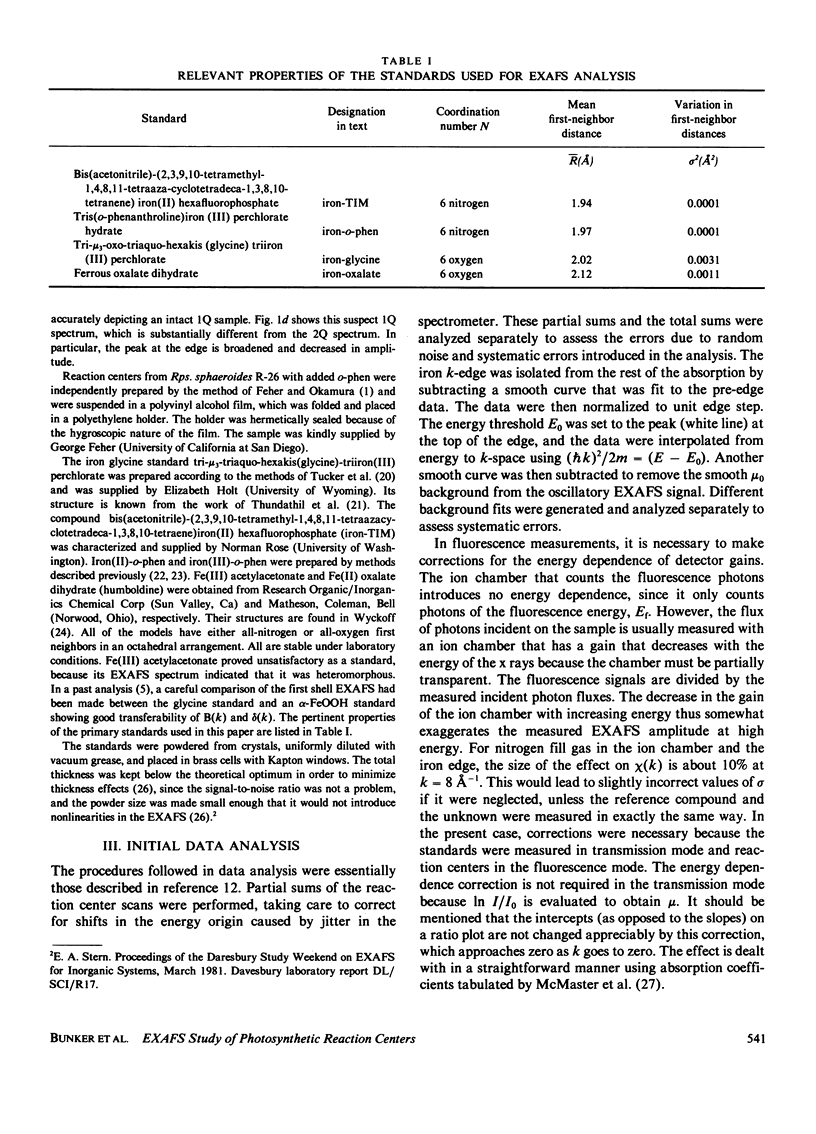
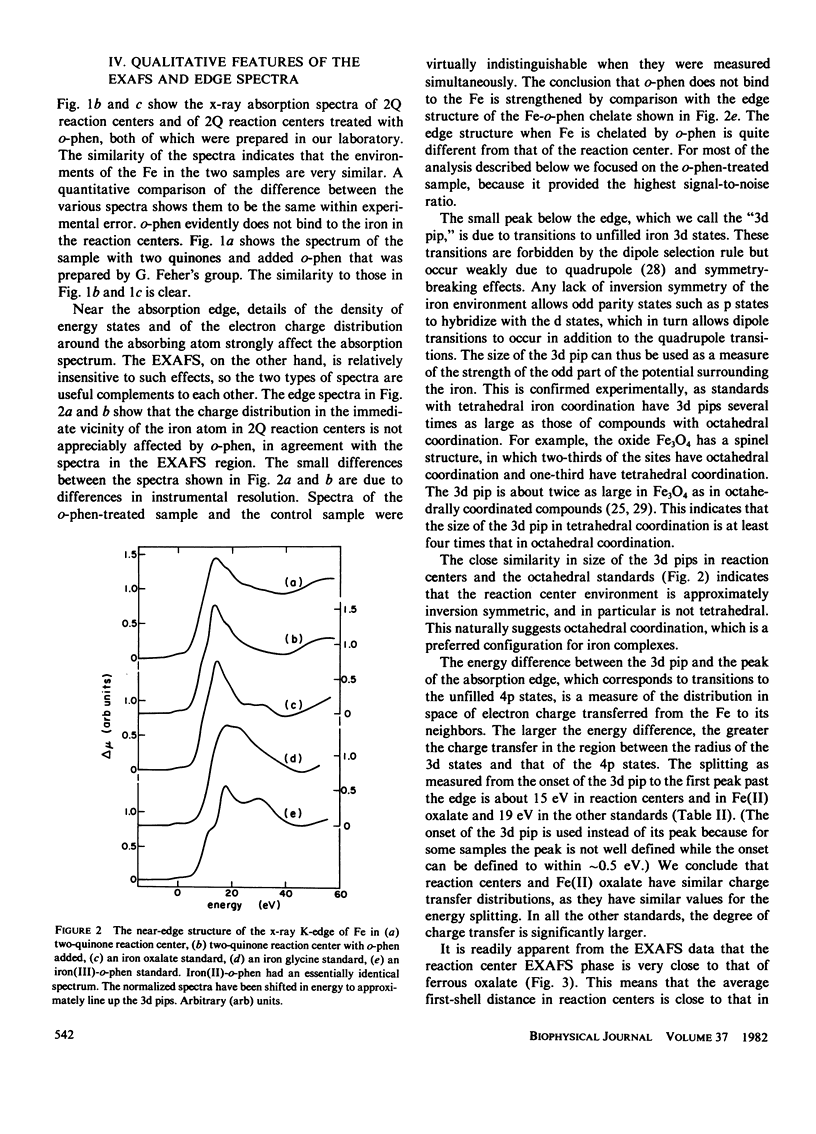
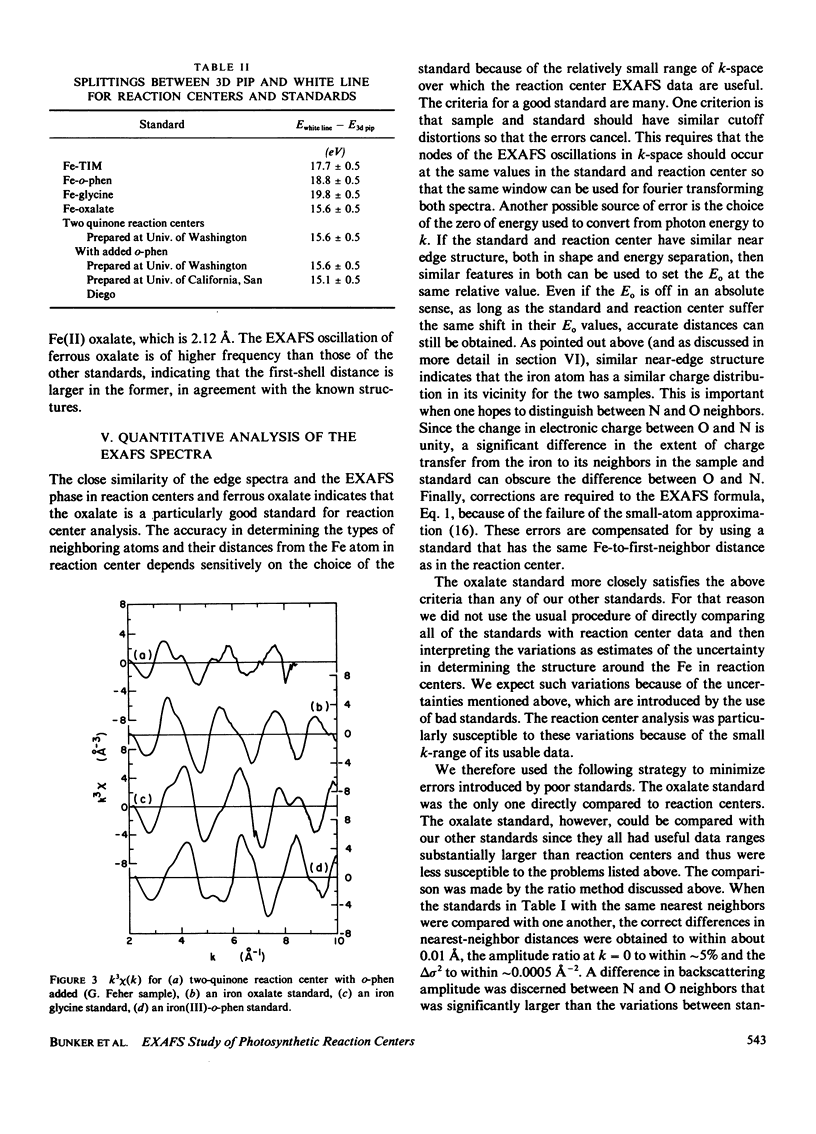
Measurements were made of the extended x-ray absorption fine structure (EXAFS) of the iron site in photosynthetic reaction centers from the bacterium Rhodopseudomonas sphaeroides. Forms with two quinones, two quinones with added o-phenanthroline, and one quinone were studied. Only the two forms containing two quinones maintained their integrity and were analyzed. The spectra show directly that the added o-phenanthroline does not chelate the iron atom. Further analysis indicates that the iron is octahedrally coordinated by nitrogen and/or oxygen atoms located at various distances, with the average value of about 2.14 A. The analysis suggests that most of the ligands are nitrogens and that three of the nitrogen ligands belong to histidine rings. This interpretation accounts for several unusual features of the EXAFS spectrum. We speculate that the quinones are bound to the histidine rings in some manner. Qualitative features of the absorption edge spectra also are discussed and are related to the Fe-ligand distance.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship R. E., Parson W. W. The involvement of iron and ubiquinone in electron transfer reactions mediated by reaction centers from photosynthetic bacteria. Biochim Biophys Acta. 1979 Mar 15;545(3):429–444. doi: 10.1016/0005-2728(79)90152-x. [DOI] [PubMed] [Google Scholar]
- Blankenship R. E., Parson W. W. The photochemical electron transfer reactions of photosynthetic bacteria and plants. Annu Rev Biochem. 1978;47:635–653. doi: 10.1146/annurev.bi.47.070178.003223. [DOI] [PubMed] [Google Scholar]
- Butler W. F., Johnston D. C., Shore H. B., Fredkin D. R., Okamura M. Y., Feher G. The electronic structure of Fe2+ in reaction centers from Rhodopseudomonas sphaeroides. I. Static magnetization measurements. Biophys J. 1980 Dec;32(3):967–992. doi: 10.1016/S0006-3495(80)85030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K., Szuts E. Z., Fleming H. Photochemical electron transport oin photosynthetic reaction centers from Rhodopseudomonas spheroides. 3. Effects of orthophenanthroline and other chemicals. Biophys J. 1972 Jan;12(1):64–79. doi: 10.1016/s0006-3495(72)86071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey Y. D., Parson W. W. Identification of ubiquinone as the secondary electron acceptor in the photosynthetic apparatus of Chromatium vinosum. Biochim Biophys Acta. 1974 Jun 28;347(3):404–416. doi: 10.1016/0005-2728(74)90079-6. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W., Case G. D. In Chromatium, a single photochemical reaction center oxidizes both cytochrome C552 and cytochrome C555. Biochim Biophys Acta. 1970;205(2):232–245. doi: 10.1016/0005-2728(70)90253-7. [DOI] [PubMed] [Google Scholar]
- Shulman G. R., Yafet Y., Eisenberger P., Blumberg W. E. Observations and interpretation of x-ray absorption edges in iron compounds and proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1384–1388. doi: 10.1073/pnas.73.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E. A. The analysis of materials by x-ray absorption. Sci Am. 1976 Apr;234(4):96–103. doi: 10.1038/scientificamerican0476-96. [DOI] [PubMed] [Google Scholar]
- Thundathil R. V., Holt E. M., Holt S. L., Watson K. J. Preparation and properties of iron (III)-amino acid complexes. 2. The crystal and molecular structure of monoclinic. Tri-mu3-oxo-triaquohexakis(glycine)triiron(III) perchlorate. J Am Chem Soc. 1977 Mar 16;99(6):1818–1823. doi: 10.1021/ja00448a024. [DOI] [PubMed] [Google Scholar]
- Tucker W. F., Asplund R. O., Holt S. L. Preparation and properties of Fe 3+-amino acid complexes; Crystalline complexes with aliphatic amino acids. Arch Biochem Biophys. 1975 Feb;166(2):433–438. doi: 10.1016/0003-9861(75)90406-3. [DOI] [PubMed] [Google Scholar]
- Valentine J. S., Sheridan R. P., Allen L. C., Kahn P. C. Coupling between oxidation state and hydrogen bond conformation in heme proteins. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1009–1013. doi: 10.1073/pnas.76.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeglio A., Clayton R. K. Kinetics of electron transfer between the primary and the secondary electron acceptor in reaction centers from Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1977 Jul 7;461(1):159–165. doi: 10.1016/0005-2728(77)90078-0. [DOI] [PubMed] [Google Scholar]



