Abstract
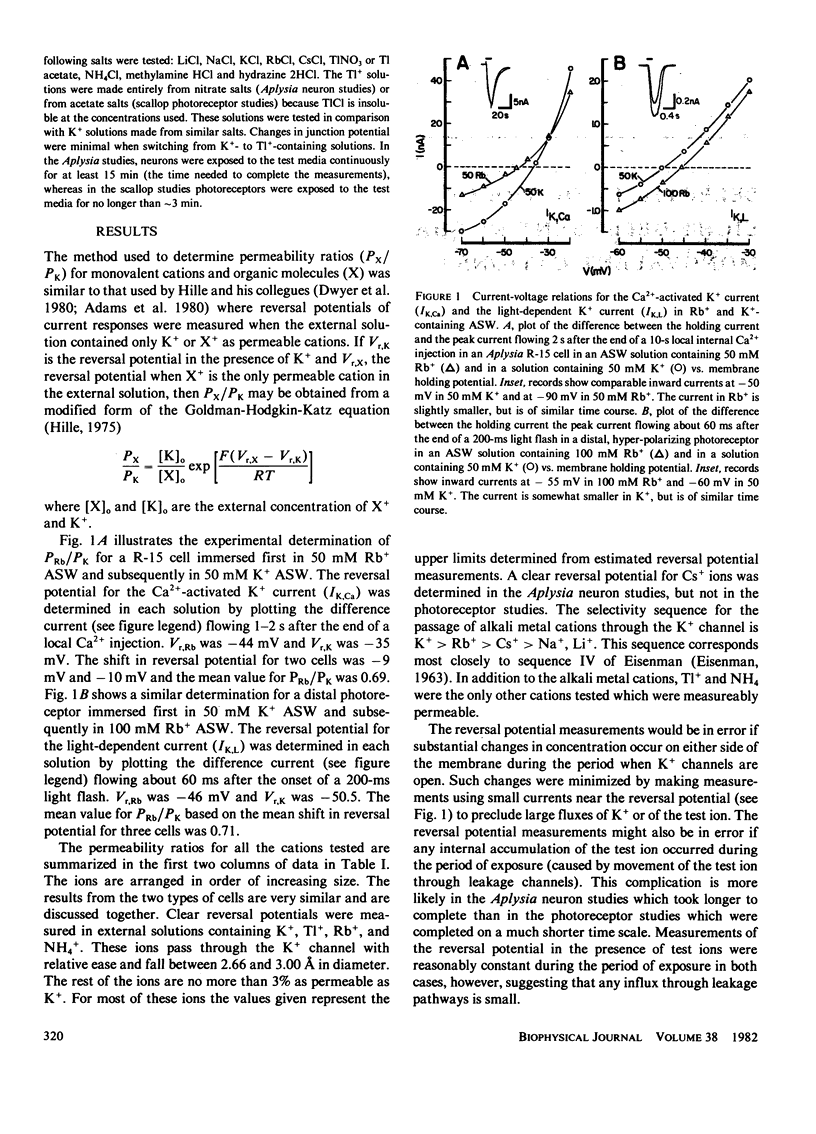
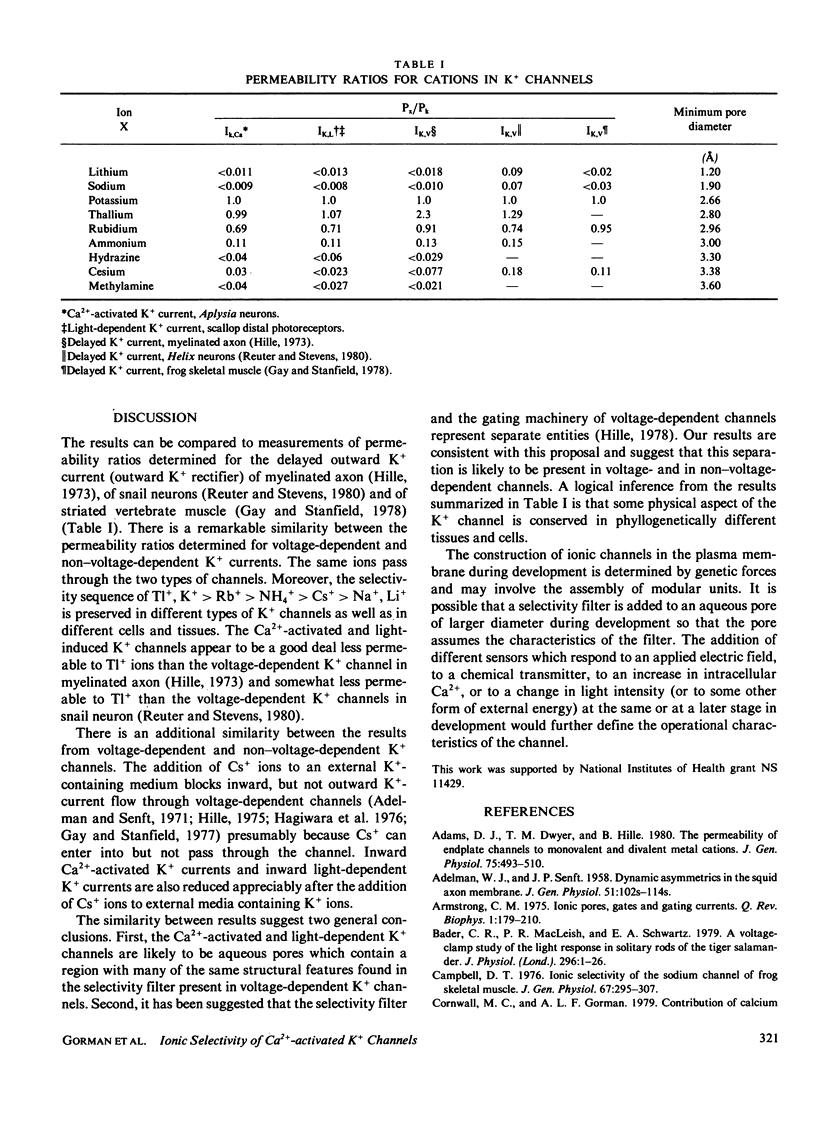
The ionic selectivity of the Ca2+-activated K+ channel of Aplysia neurons and of the light-dependent K+ channel of Pecten photoreceptors to metal and organic cations was studied. The selectivity sequence determined from reversal potential measurements is T1+ K+ > Rb+ > NH+4 > Cs+ > Na+, Li+ and is identical to the sequence determined previously for voltage-dependent K+ channels in a variety of tissues. Our results suggest that some physical aspect of the K+ channel is conserved in phyllogenetically different tissues and cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Senft J. P. Dynamic asymmetries in the squid axon membrane. J Gen Physiol. 1968 May;51(5 Suppl):102S+–102S+. [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. T. Ionic selectivity of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976 Mar;67(3):295–307. doi: 10.1085/jgp.67.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer T. M., Adams D. J., Hille B. The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol. 1980 May;75(5):469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. Cs(+) causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977 May 12;267(5607):169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. The selectivity of the delayed potassium conductance of frog skeletal muscle fibers. Pflugers Arch. 1978 Dec 28;378(2):177–179. doi: 10.1007/BF00584453. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Serotonin: two different inhibitory actions on snail neurons. Science. 1971 Mar 26;171(3977):1252–1254. doi: 10.1126/science.171.3977.1252. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A. Internal effects of divalent cations on potassium permeability in molluscan neurones. J Physiol. 1979 Nov;296:393–410. doi: 10.1113/jphysiol.1979.sp013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., McReynolds J. S. Ionic effects on the membrane potential of hyperpolarizing photoreceptors in scallop retina. J Physiol. 1978 Feb;275:345–355. doi: 10.1113/jphysiol.1978.sp012193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., TRAUTWEIN W. Vagal and sympathetic effects on the pacemaker fibers in the sinus venosus of the heart. J Gen Physiol. 1956 May 20;39(5):715–733. doi: 10.1085/jgp.39.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic channels in excitable membranes. Current problems and biophysical approaches. Biophys J. 1978 May;22(2):283–294. doi: 10.1016/S0006-3495(78)85489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. Y., Catterall W. A., Ehrenstein G. Selectivity of cations and nonelectrolytes for acetylcholine-activated channels in cultured muscle cells. J Gen Physiol. 1978 Apr;71(4):397–410. doi: 10.1085/jgp.71.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F. Ion conductance and ion selectivity of potassium channels in snail neurones. J Membr Biol. 1980 Dec 15;57(2):103–118. doi: 10.1007/BF01868997. [DOI] [PubMed] [Google Scholar]



