Abstract
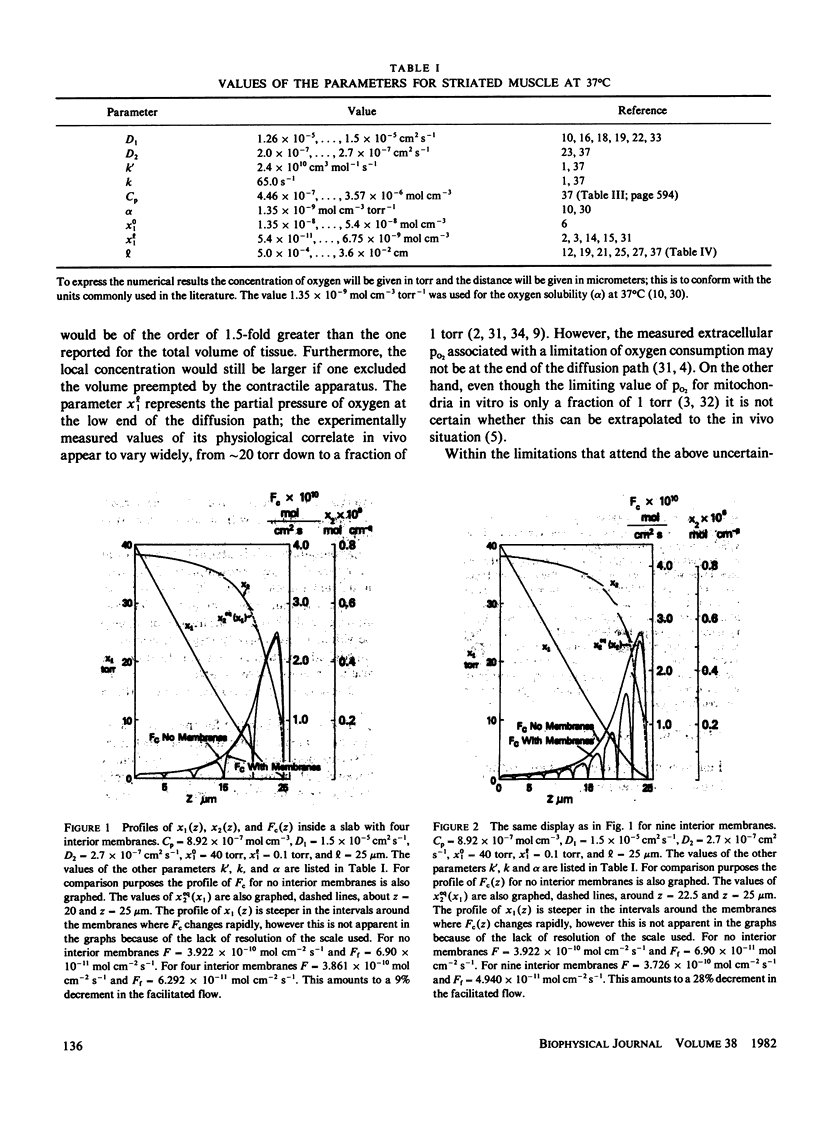
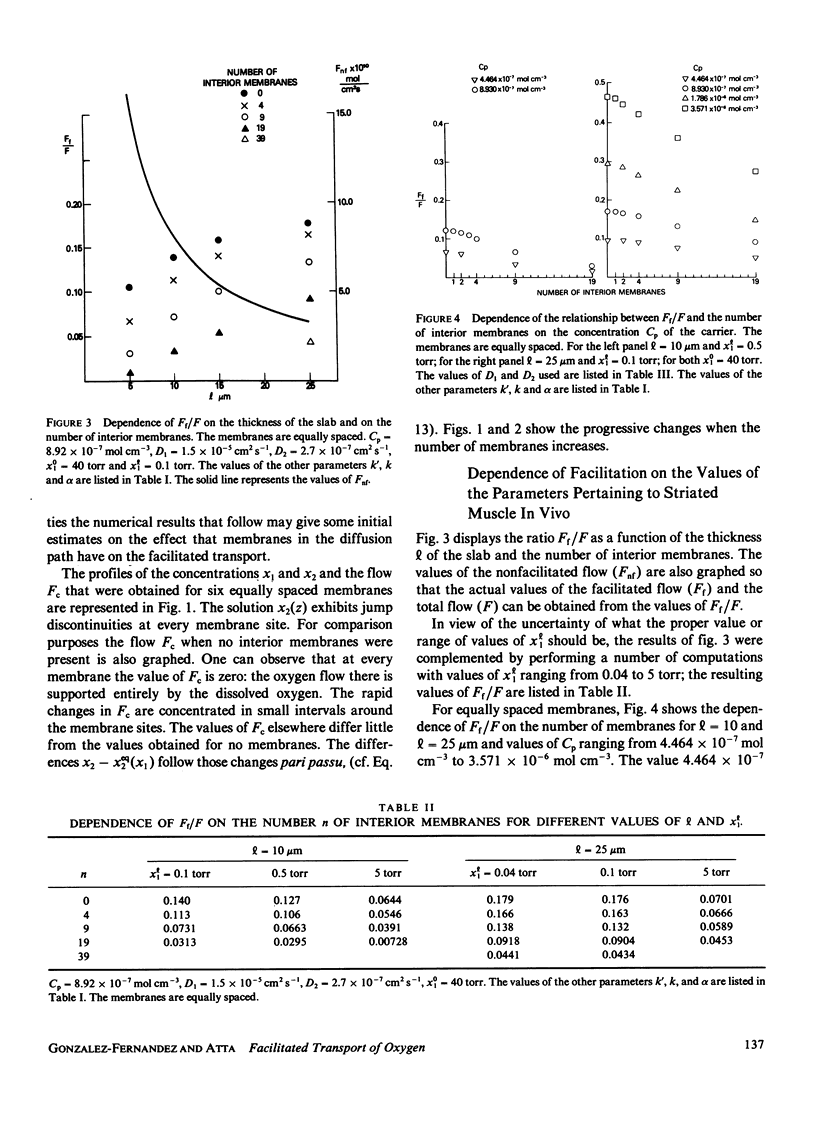
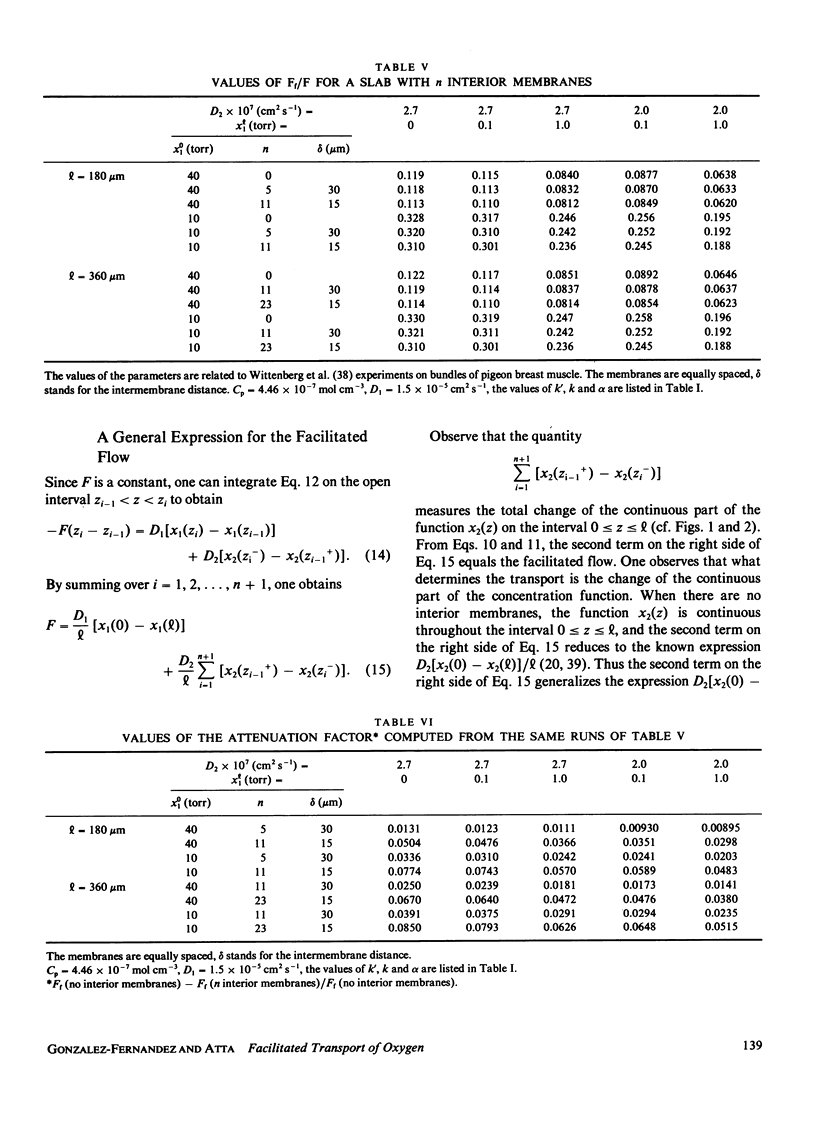
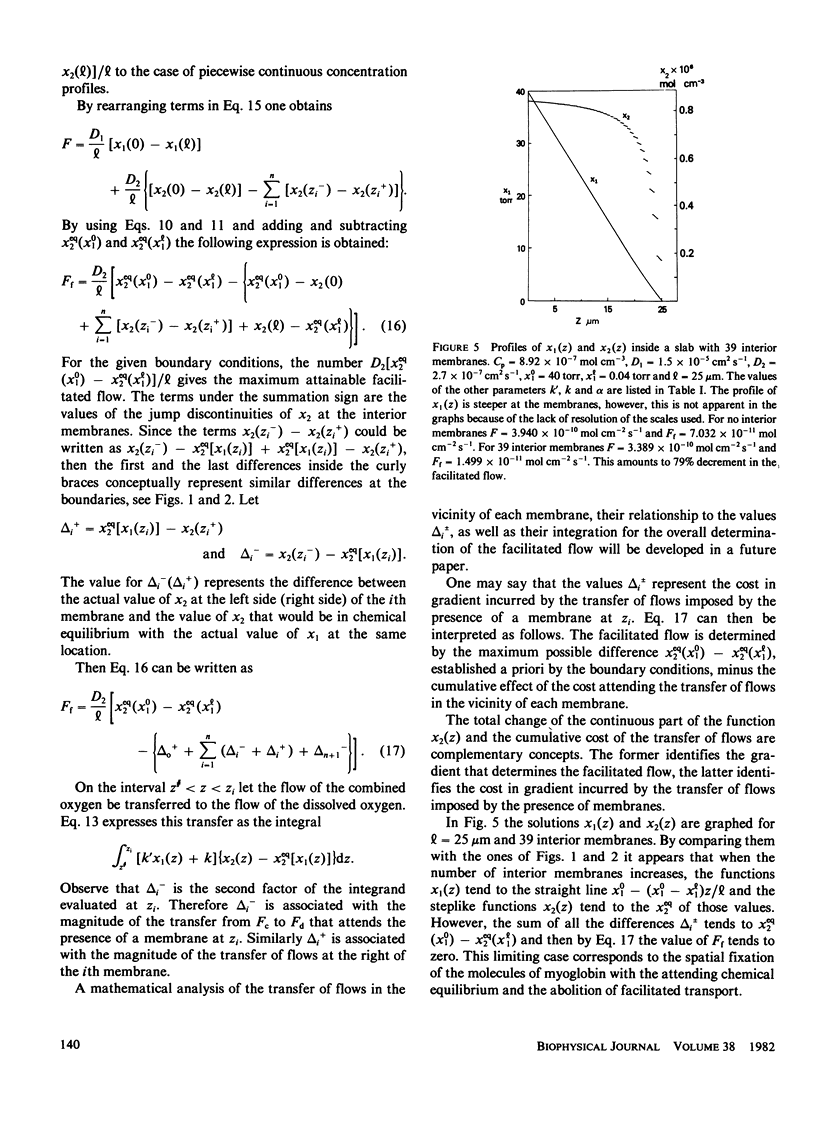
Most of the experimental observations on facilitated transport have been done with millipore filters, and all the theoretical studies have assumed homogeneous spatial properties. In striated muscle there exist membranes that may impede the diffusion of the carrier myoglobin. In this paper a theoretical study is undertaken to analyze the transport in the presence of membranes in the diffusion path. For the numerical computations physiologically relevant values of the parameters were chosen. The numerical results indicate that the presence of membranes tends to decrease the facilitation. For the nonlinear chemical kinetics of the reaction of oxygen with the carrier, this decrement also depends on the location of the membranes. At the higher oxygen concentration side of each membrane the flow of combined oxygen is transferred to the flow of dissolved oxygen. The reverse process occurs at the lower concentration side. Jump discontinuities of the concentration of the oxygen-carrier compound at each membrane are associated with these transfers. The decrement of facilitation is due to the cumulative effect of these jump discontinuities.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duling B. R. Effects of potassium ion on the microcirculation of the hamster. Circ Res. 1975 Sep;37(3):325–332. doi: 10.1161/01.res.37.3.325. [DOI] [PubMed] [Google Scholar]
- Duling B. R. Microvascular responses to alterations in oxygen tension. Circ Res. 1972 Oct;31(4):481–489. doi: 10.1161/01.res.31.4.481. [DOI] [PubMed] [Google Scholar]
- GROTE J., THEWS G. [Requirements for the oxygen supply of heart muscle tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1962;276:142–165. [PubMed] [Google Scholar]
- Goldfischer S. The cytochemical localization of myoglobin in striated muscle of man and walrus. J Cell Biol. 1967 Jul;34(1):398–403. doi: 10.1083/jcb.34.1.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. J., Duling B. R. Role of oxygen in arteriolar functional vasodilation in hamster striated muscle. Am J Physiol. 1978 Nov;235(5):H505–H515. doi: 10.1152/ajpheart.1978.235.5.H505. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., OLSON C. B. RELATIONS BETWEEN STRUCTURE AND FUNCTION IN THE DESIGN OF SKELETAL MUSCLES. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- Hearon J. Z. Distribution of conserved species in diffusion-reaction systems. Bull Math Biol. 1973 Feb-Apr;35(1):59–67. doi: 10.1007/BF02558794. [DOI] [PubMed] [Google Scholar]
- James N. T. Histochemical demonstration of myoglobin in skeletal muscle fibres and muscle spindles. Nature. 1968 Sep 14;219(5159):1174–1175. doi: 10.1038/2191174a0. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F., Stainsby W. N. Oxidation of NADH during contractions of circulated mammalian skeletal muscle. Respir Physiol. 1968 May;4(3):292–300. doi: 10.1016/0034-5687(68)90035-2. [DOI] [PubMed] [Google Scholar]
- Kawashiro T., Nüsse W., Scheid P. Determination of diffusivity of oxygen and carbon dioxide in respiring tissue: results in rat skeletal muscle. Pflugers Arch. 1975 Sep 9;359(3):231–251. doi: 10.1007/BF00587382. [DOI] [PubMed] [Google Scholar]
- Kreuzer F., Hoofd L. J. Facilitated diffusion of oxygen in the presence of hemoglobin. Respir Physiol. 1970 Mar;8(3):280–302. doi: 10.1016/0034-5687(70)90037-x. [DOI] [PubMed] [Google Scholar]
- Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919 May 20;52(6):409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A. The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J Physiol. 1919 May 20;52(6):391–408. doi: 10.1113/jphysiol.1919.sp001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutchai H., Jacquez J. A., Mather F. J. Nonequilibrium facilitated oxygen transport in hemoglobin solution. Biophys J. 1970 Jan;10(1):38–54. doi: 10.1016/S0006-3495(70)86284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS W. W., HONIG C. R. NUMBER AND DISTRIBUTION OF CAPILLARIES AS DETERMINANTS OF MYOCARDIAL OXYGEN TENSION. Am J Physiol. 1964 Sep;207:653–660. doi: 10.1152/ajplegacy.1964.207.3.653. [DOI] [PubMed] [Google Scholar]
- Mahler M. Diffusion and consumption of oxygen in the resting frog sartorius muscle. J Gen Physiol. 1978 May;71(5):533–557. doi: 10.1085/jgp.71.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll W. The diffusion coefficient of myoglobin in muscle homogenate. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;299(3):247–251. doi: 10.1007/BF00362587. [DOI] [PubMed] [Google Scholar]
- Morita S., Cassens R. G., Briskey E. J. Localization of myoglobin in striated muscle of the domestic pig; benzidine and NADH2-TR reactions. Stain Technol. 1969 Nov;44(6):283–286. doi: 10.3109/10520296909063367. [DOI] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- ROMANUL F. C. CAPILLARY SUPPLY AND METABOLISM OF MUSCLE FIBERS. Arch Neurol. 1965 May;12:497–509. doi: 10.1001/archneur.1965.00460290053007. [DOI] [PubMed] [Google Scholar]
- Rubinow S. I., Dembo M. The facilitated diffusion of oxygen by hemoglobin and myoglobin. Biophys J. 1977 Apr;18(1):29–42. doi: 10.1016/S0006-3495(77)85594-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOLANDER P. F. Oxygen transport through hemoglobin solutions. Science. 1960 Feb 26;131(3400):585–590. doi: 10.1126/science.131.3400.585. [DOI] [PubMed] [Google Scholar]
- Starlinger H., Lübbers D. W. Polarographic measurements of the oxygen pressure performed simultaneously with optical measurements of the redox state of the respiratory chain in suspensions of mitochondria under steady-state conditions at low oxygen tensions. Pflugers Arch. 1973;341(1):15–22. doi: 10.1007/BF00587326. [DOI] [PubMed] [Google Scholar]
- Whalen W. J., Nair P. Skeletal muscle PO2: effect of inhaled and topically applied O2 and CO2. Am J Physiol. 1970 Apr;218(4):973–980. doi: 10.1152/ajplegacy.1970.218.4.973. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Wittenberg J. B., Caldwell P. R. Role of myoglobin in the oxygen supply to red skeletal muscle. J Biol Chem. 1975 Dec 10;250(23):9038–9043. [PubMed] [Google Scholar]
- Wittenberg J. B. Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiol Rev. 1970 Oct;50(4):559–636. doi: 10.1152/physrev.1970.50.4.559. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B. The molecular mechanism of hemoglobin-facilitated oxygen diffusion. J Biol Chem. 1966 Jan 10;241(1):104–114. [PubMed] [Google Scholar]
- Wyman J. Facilitated diffusion and the possible role of myoglobin as a transport mechanism. J Biol Chem. 1966 Jan 10;241(1):115–121. [PubMed] [Google Scholar]


