Abstract
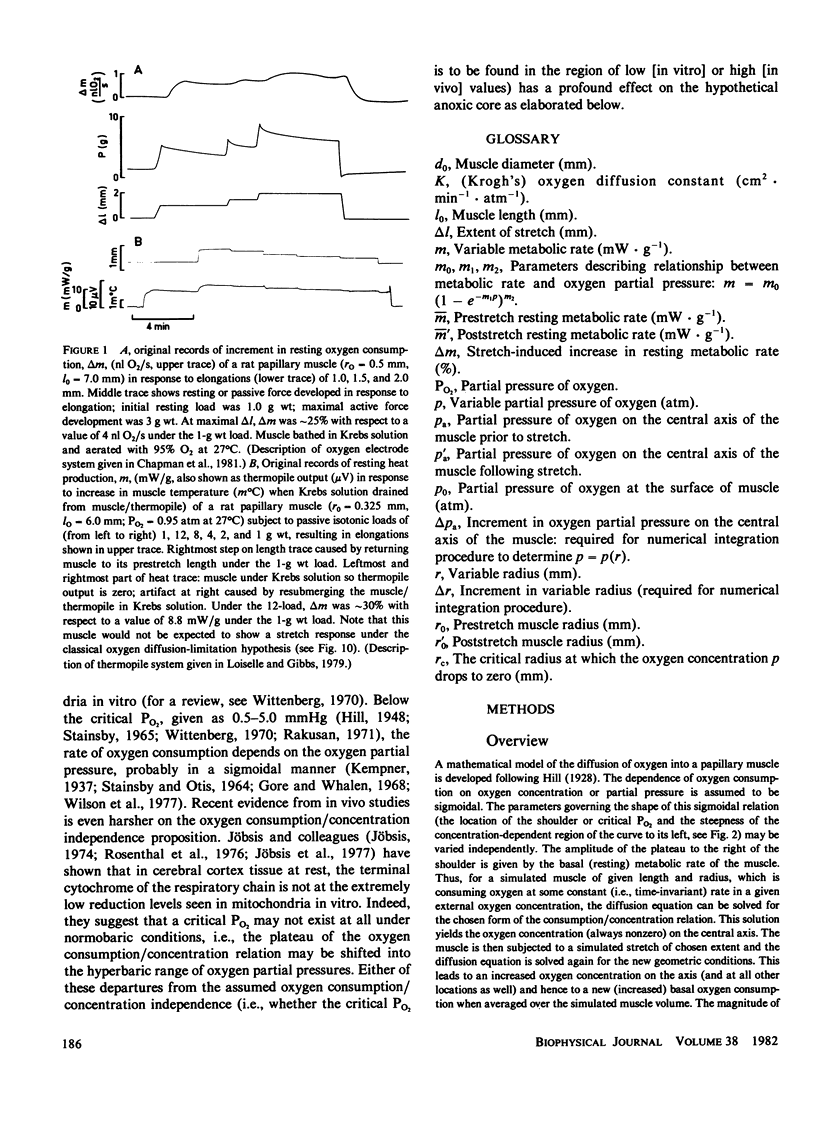
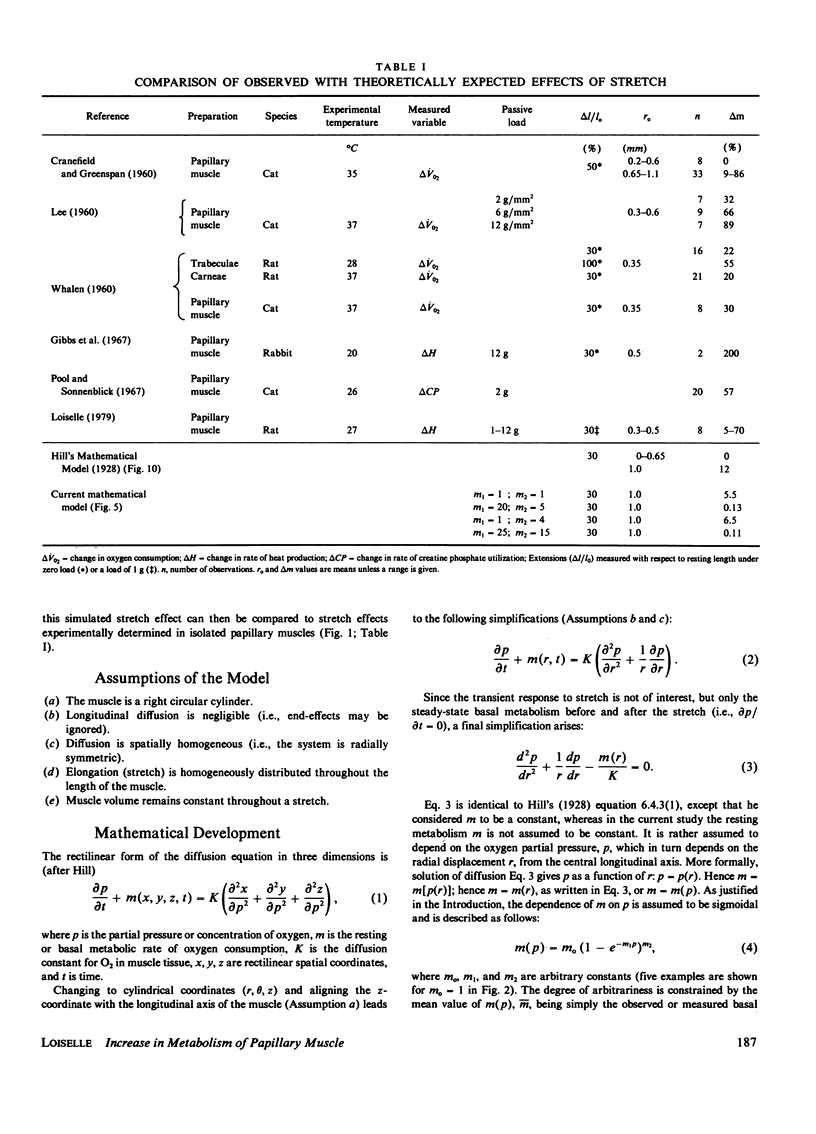
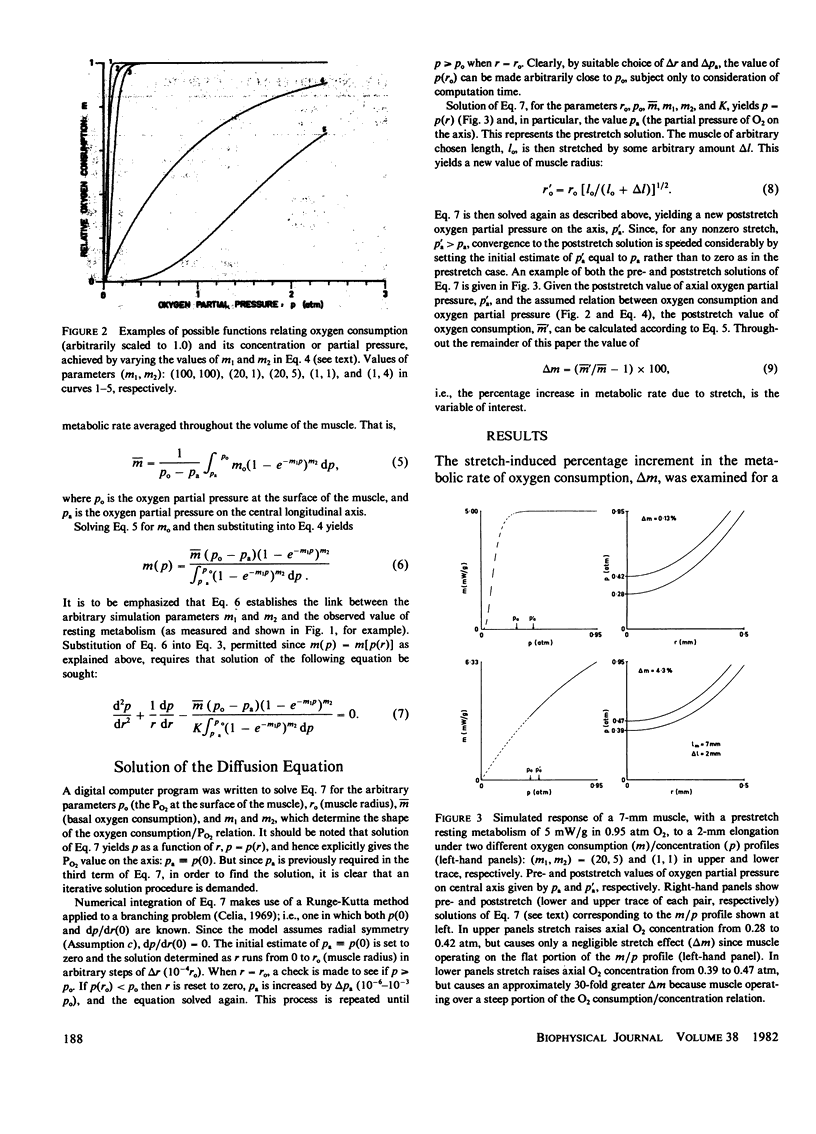
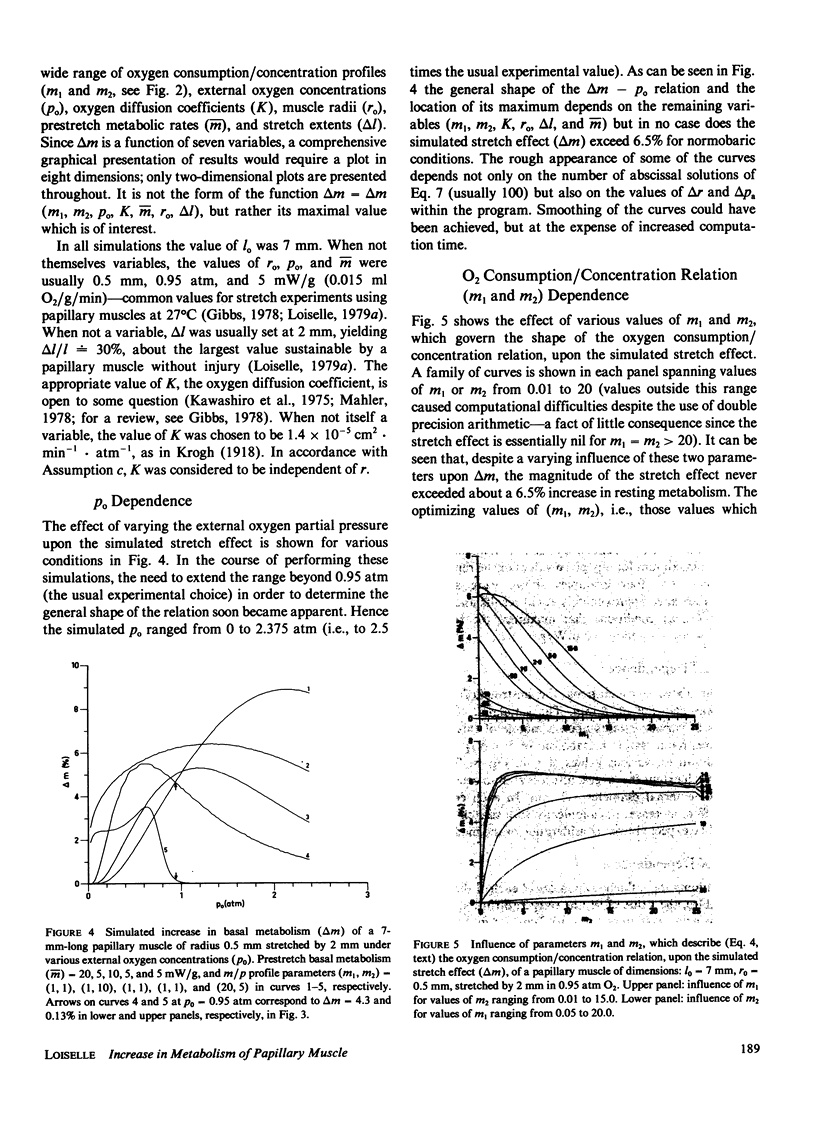
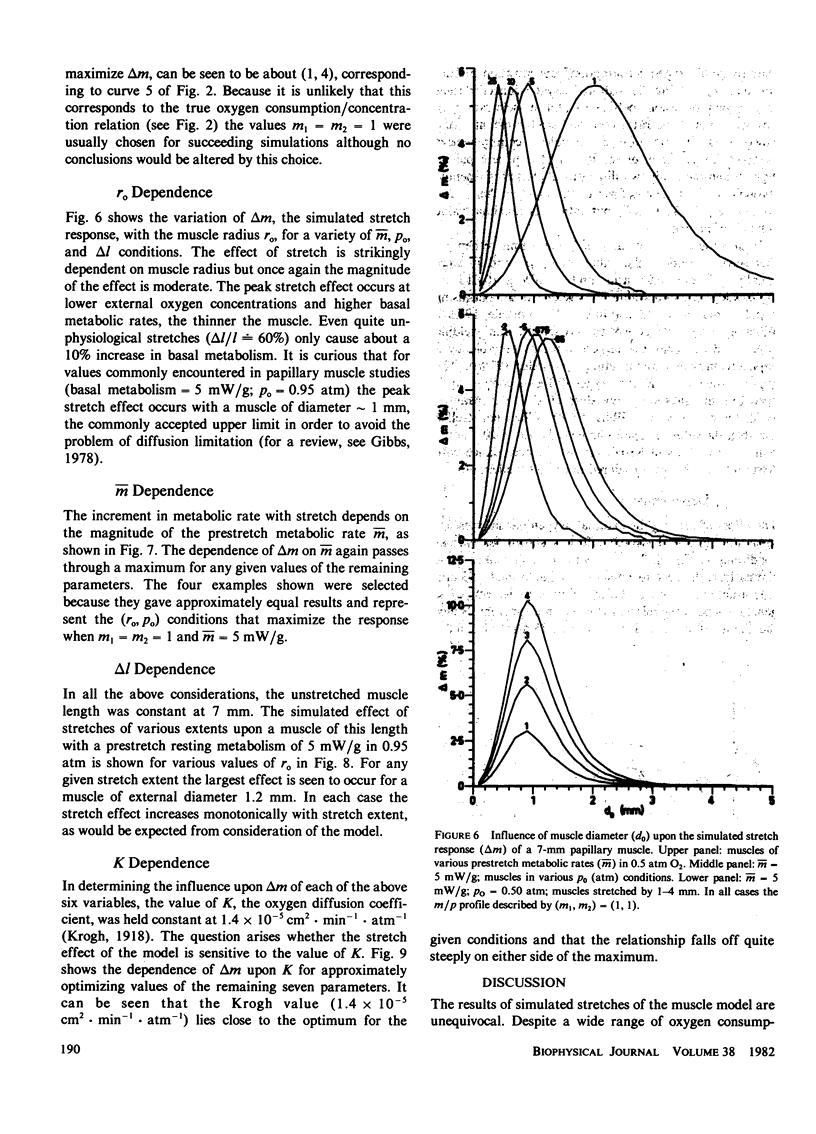
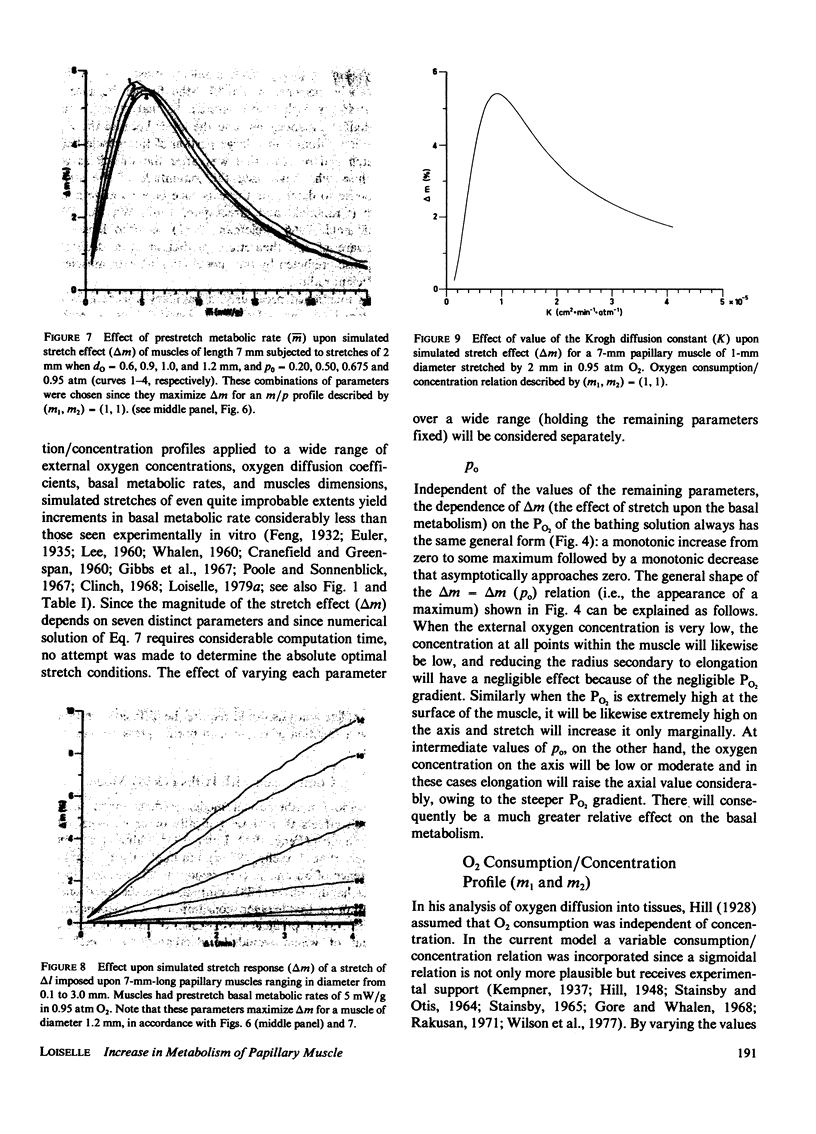
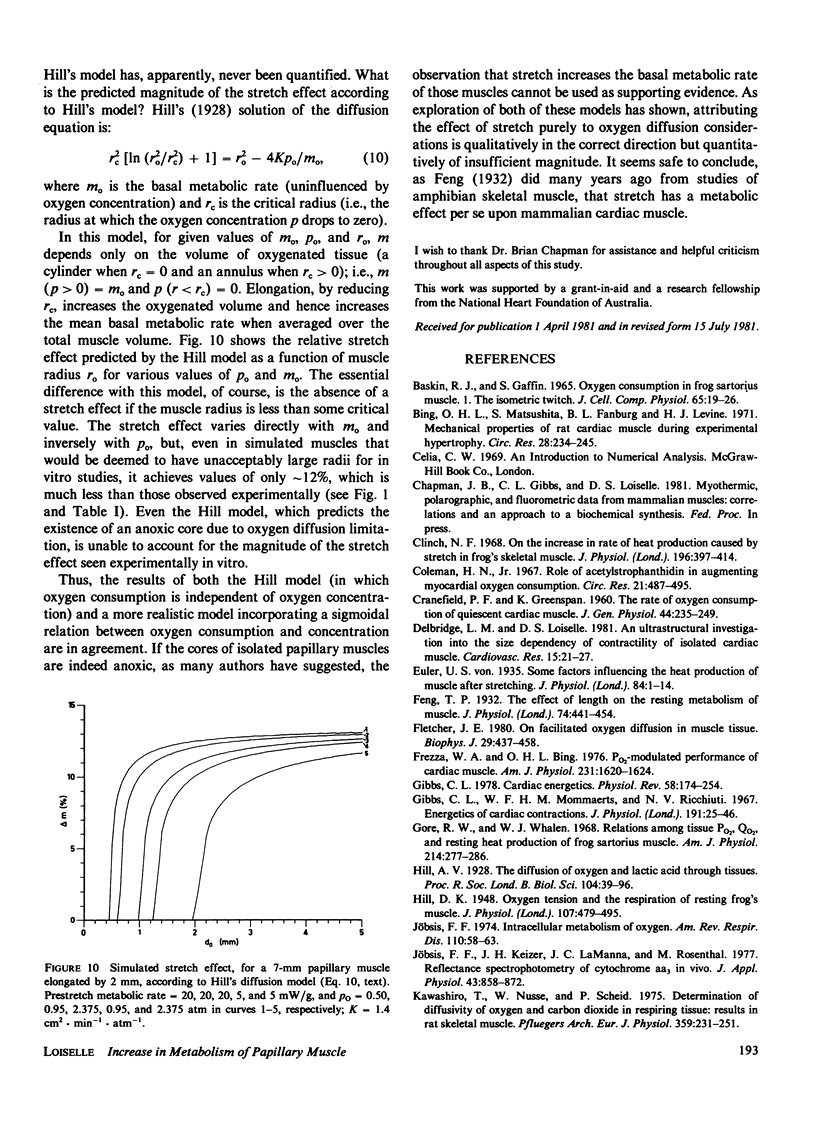
A mathematical model of oxygen diffusion into quiescent papillary muscles in vitro is developed. The model incorporates a continuous sigmoidal function relating the rate of oxygen consumption and the partial pressure of oxygen within the tissue. The behavior of the model is explored over a wide range of external oxygen partial pressures, oxygen consumption/partial pressure relations, oxygen diffusivities, muscle dimensions, and resting metabolic rates, while the muscle is subjected to stimulated stretches of various extents in order to test the assertion that the stretch-induced increase in basal metabolic rate observed experimentally implies the existence of an anoxic core region of papillary muscles in vitro. The model predicts the existence of an oxygen diffusion-mediated stretch response of resting papillary muscle metabolism, but one which is quantitatively insignificant compared with experimentally observed values. The classic Hill diffusion model which explicitly predicts an anoxic core, likewise predicts stretch effects of magnitudes smaller than those frequently observed. It is concluded that the increment in basal metabolism of papillary muscles subjected to stretch in vivo cannot be taken as evidence of oxygen diffusion limitation in unstretched preparations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASKIN R. J., GAFFIN S. OXYGEN CONSUMPTION IN FROG SARTORIUS MUSCLE. I. THE ISOMETRIC TWITCH. J Cell Physiol. 1965 Feb;65:19–25. doi: 10.1002/jcp.1030650104. [DOI] [PubMed] [Google Scholar]
- Bing O. H., Matsushita S., Fanburg B. L., Levine H. J. Mechanical properties of rat cardiac muscle during experimental hypertrophy. Circ Res. 1971 Feb;28(2):234–245. doi: 10.1161/01.res.28.2.234. [DOI] [PubMed] [Google Scholar]
- CRANEFIELD P. F., GREENSPAN K. The rate of oxygen uptake of quiescent cardiac muscle. J Gen Physiol. 1960 Nov;44:235–249. doi: 10.1085/jgp.44.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinch N. F. On the increase in rate of heat production caused by stretch in frog's skeletal muscle. J Physiol. 1968 May;196(2):397–414. doi: 10.1113/jphysiol.1968.sp008514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman H. N., 3rd Role of acetylstrophanthidin in augmenting myocardial oxygen consumption. Relation of increased O-2 consumption to changes in velocity of contraction. Circ Res. 1967 Oct;21(4):487–495. doi: 10.1161/01.res.21.4.487. [DOI] [PubMed] [Google Scholar]
- Delbridge L. M., Loiselle D. S. An ultrastructural investigation into the size dependency of contractility of isolated cardiac muscle. Cardiovasc Res. 1981 Jan;15(1):21–27. doi: 10.1093/cvr/15.1.21. [DOI] [PubMed] [Google Scholar]
- Feng T. P. The effect of length on the resting metabolism of muscle. J Physiol. 1932 Apr 26;74(4):441–454. doi: 10.1113/jphysiol.1932.sp002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. E. On facilitated oxygen diffusion in muscle tissues. Biophys J. 1980 Mar;29(3):437–458. doi: 10.1016/S0006-3495(80)85145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezza W. A., Bing O. H. PO2-modulated performance of cardiac muscle. Am J Physiol. 1976 Nov;231(5 Pt 1):1620–1624. doi: 10.1152/ajplegacy.1976.231.5.1620. [DOI] [PubMed] [Google Scholar]
- Gibbs C. L. Cardiac energetics. Physiol Rev. 1978 Jan;58(1):174–254. doi: 10.1152/physrev.1978.58.1.174. [DOI] [PubMed] [Google Scholar]
- Gibbs C. L., Mommaerts W. F., Ricchiuti N. V. Energetics of cardiac contractions. J Physiol. 1967 Jul;191(1):25–46. doi: 10.1113/jphysiol.1967.sp008235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore R. W., Whalen W. J. Relations among tissue PO2, QO2, and resting heat production of frog sartorius muscle. Am J Physiol. 1968 Feb;214(2):277–286. doi: 10.1152/ajplegacy.1968.214.2.277. [DOI] [PubMed] [Google Scholar]
- Hill D. K. Oxygen tension and the respiration of resting frog's muscle. J Physiol. 1948 Sep 30;107(4):479–495. doi: 10.1113/jphysiol.1948.sp004293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis F. F., Keizer J. H., LaManna J. C., Rosenthal M. Reflectance spectrophotometry of cytochrome aa3 in vivo. J Appl Physiol Respir Environ Exerc Physiol. 1977 Nov;43(5):858–872. doi: 10.1152/jappl.1977.43.5.858. [DOI] [PubMed] [Google Scholar]
- KELLY J. J., Jr, HOFFMAN B. F. Mechanical activity of rat papillary muscle. Am J Physiol. 1960 Jul;199:157–162. doi: 10.1152/ajplegacy.1960.199.1.157. [DOI] [PubMed] [Google Scholar]
- Kawashiro T., Nüsse W., Scheid P. Determination of diffusivity of oxygen and carbon dioxide in respiring tissue: results in rat skeletal muscle. Pflugers Arch. 1975 Sep 9;359(3):231–251. doi: 10.1007/BF00587382. [DOI] [PubMed] [Google Scholar]
- Krogh A. The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J Physiol. 1919 May 20;52(6):391–408. doi: 10.1113/jphysiol.1919.sp001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENTINI E. A. MYOCARDIAL DEVELOPED TENSION AND OXYGEN SUPPLY. Am J Physiol. 1964 Aug;207:341–346. doi: 10.1152/ajplegacy.1964.207.2.341. [DOI] [PubMed] [Google Scholar]
- Loiselle D. S., Gibbs C. L. Species differences in cardiac energetics. Am J Physiol. 1979 Jul;237(1):H90–H98. doi: 10.1152/ajpheart.1979.237.1.H90. [DOI] [PubMed] [Google Scholar]
- Loiselle D. S. The effects of temperature on the energetics of rat papillary muscle. Pflugers Arch. 1979 Mar 16;379(2):173–180. doi: 10.1007/BF00586944. [DOI] [PubMed] [Google Scholar]
- Mahler M. Diffusion and consumption of oxygen in the resting frog sartorius muscle. J Gen Physiol. 1978 May;71(5):533–557. doi: 10.1085/jgp.71.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R. H., Jr Developed tension: a major determinant of myocardial oxygen consumption. Am J Physiol. 1966 Feb;210(2):351–356. doi: 10.1152/ajplegacy.1966.210.2.351. [DOI] [PubMed] [Google Scholar]
- Pool P. E., Sonnenblick E. H. The mechanochemistry of cardiac muscle. I. The isometric contraction. J Gen Physiol. 1967 Mar;50(4):951–965. doi: 10.1085/jgp.50.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M., Lamanna J. C., Jöbsis F. F., Levasseur J. E., Kontos H. A., Patterson J. L. Effects of respiratory gases on cytochrome A in intact cerebral cortex: is there a critical Po2? Brain Res. 1976 May 21;108(1):143–154. doi: 10.1016/0006-8993(76)90170-0. [DOI] [PubMed] [Google Scholar]
- STAINSBY W. N., OTIS A. B. BLOOD FLOW, BLOOD OXYGEN TENSION, OXYGEN UPTAKE, AND OXYGEN TRANSPORT IN SKELETAL MUSCLE. Am J Physiol. 1964 Apr;206:858–866. doi: 10.1152/ajplegacy.1964.206.4.858. [DOI] [PubMed] [Google Scholar]
- Snow T. R., Bressler P. B. Oxygen sufficiency in working rabbit papillary muscle at 35 degrees C. J Mol Cell Cardiol. 1977 Jul;9(7):595–604. doi: 10.1016/s0022-2828(77)80373-8. [DOI] [PubMed] [Google Scholar]
- V Euler U. S. Some factors influencing the heat production of muscle after stretching. J Physiol. 1935 Apr 26;84(1):1–14. doi: 10.1113/jphysiol.1935.sp003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHALEN W. J., DERNBERG E., JENDEN D. J. Interaction of stretch and ryanodine on oxygen uptake in the rat diaphragm. Am J Physiol. 1958 Aug;194(2):359–362. doi: 10.1152/ajplegacy.1958.194.2.359. [DOI] [PubMed] [Google Scholar]
- WHALEN W. J. Some factors influencing O2 consumption of isolated heart muscle. Am J Physiol. 1960 Jun;198:1153–1156. doi: 10.1152/ajplegacy.1960.198.6.1153. [DOI] [PubMed] [Google Scholar]
- WHALEN W. J. The relation of work and oxygen consumption in isolated strips of cat and rat myocardium. J Physiol. 1961 Jun;157:1–17. doi: 10.1113/jphysiol.1961.sp006701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F., Erecińska M., Drown C., Silver I. A. Effect of oxygen tension on cellular energetics. Am J Physiol. 1977 Nov;233(5):C135–C140. doi: 10.1152/ajpcell.1977.233.5.C135. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B. Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiol Rev. 1970 Oct;50(4):559–636. doi: 10.1152/physrev.1970.50.4.559. [DOI] [PubMed] [Google Scholar]
- Yamada K. The increase in the rate of heat production of frog's skeletal muscle caused by hypertonic solutions. J Physiol. 1970 May;208(1):49–64. doi: 10.1113/jphysiol.1970.sp009105. [DOI] [PMC free article] [PubMed] [Google Scholar]



