Abstract
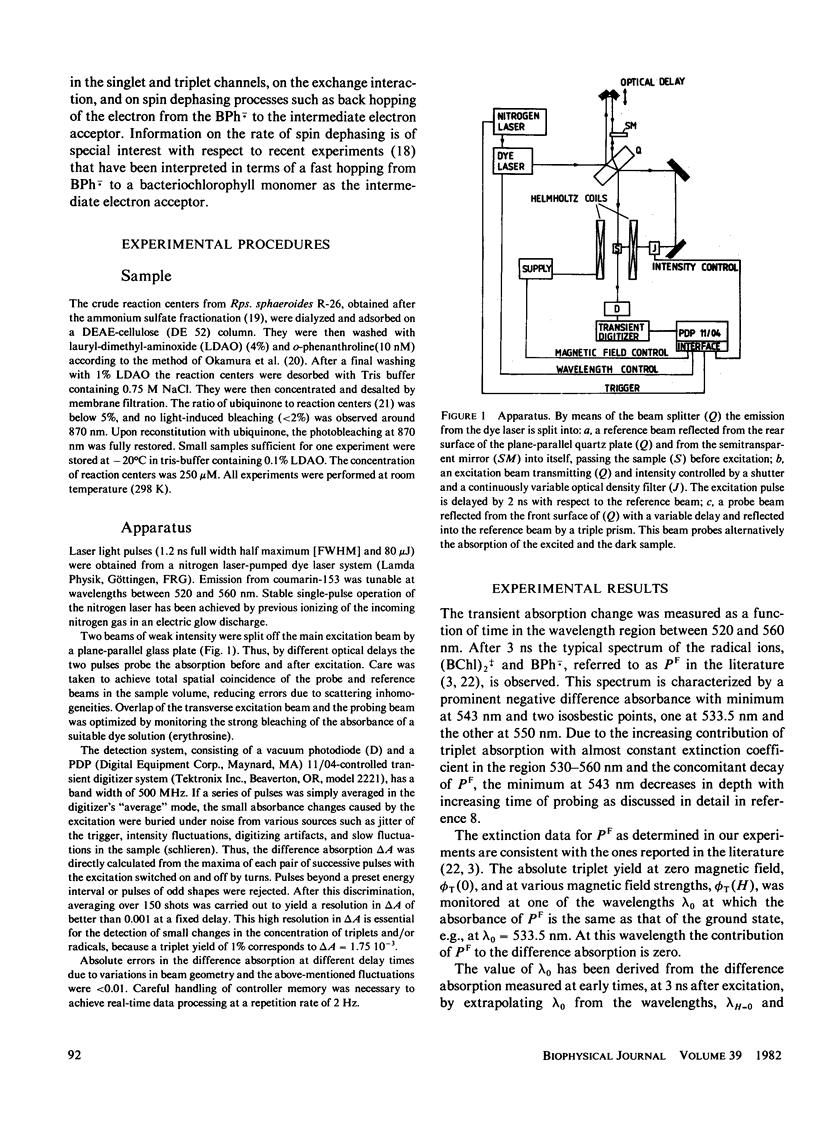
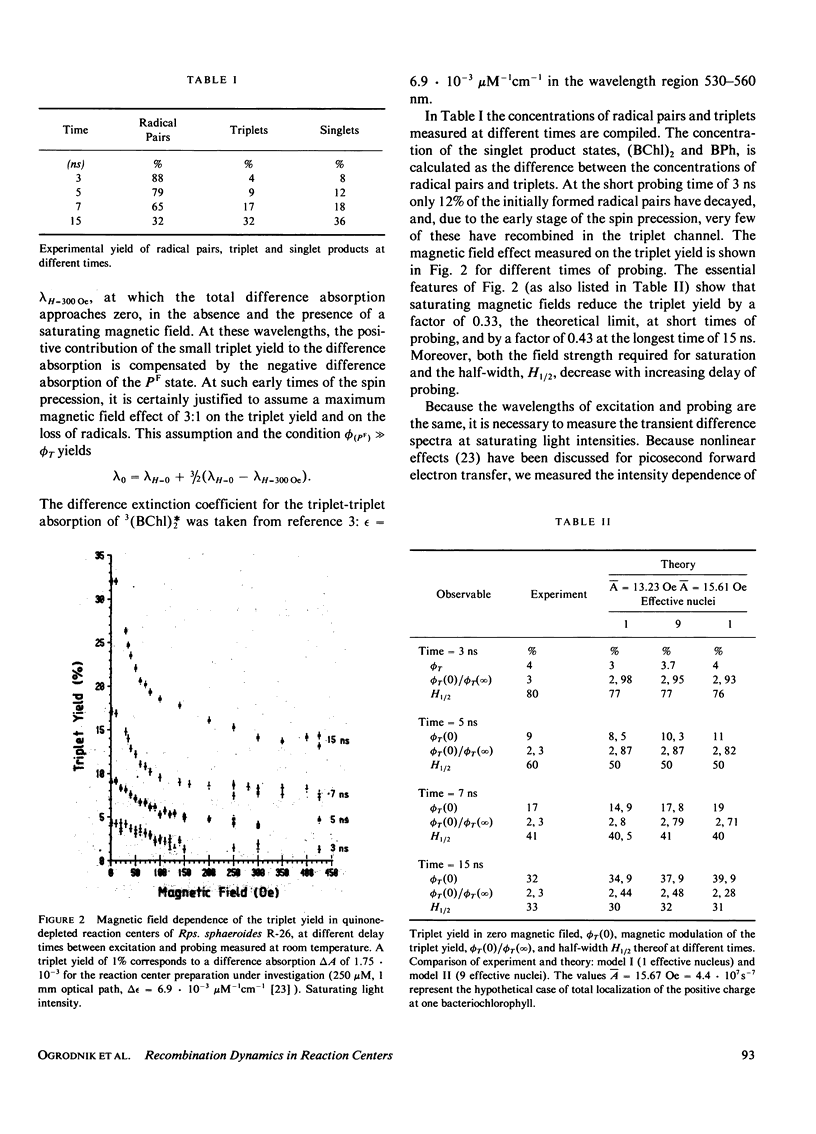
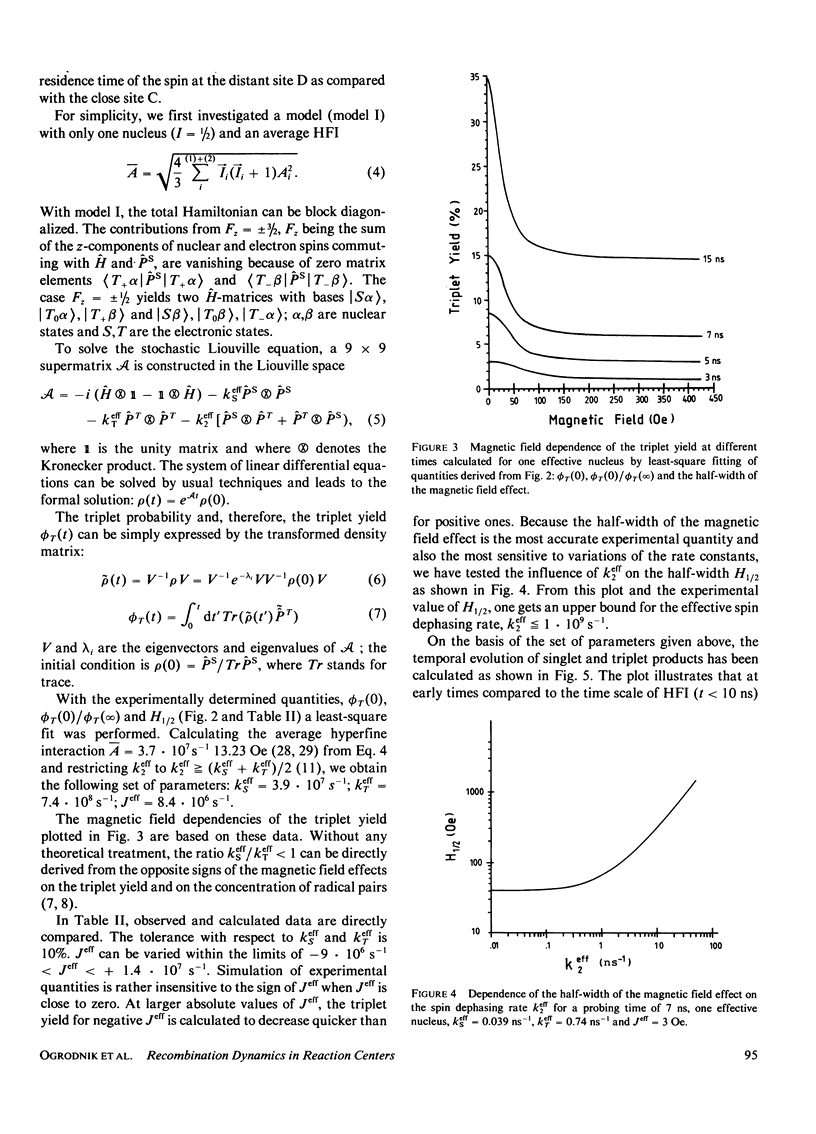
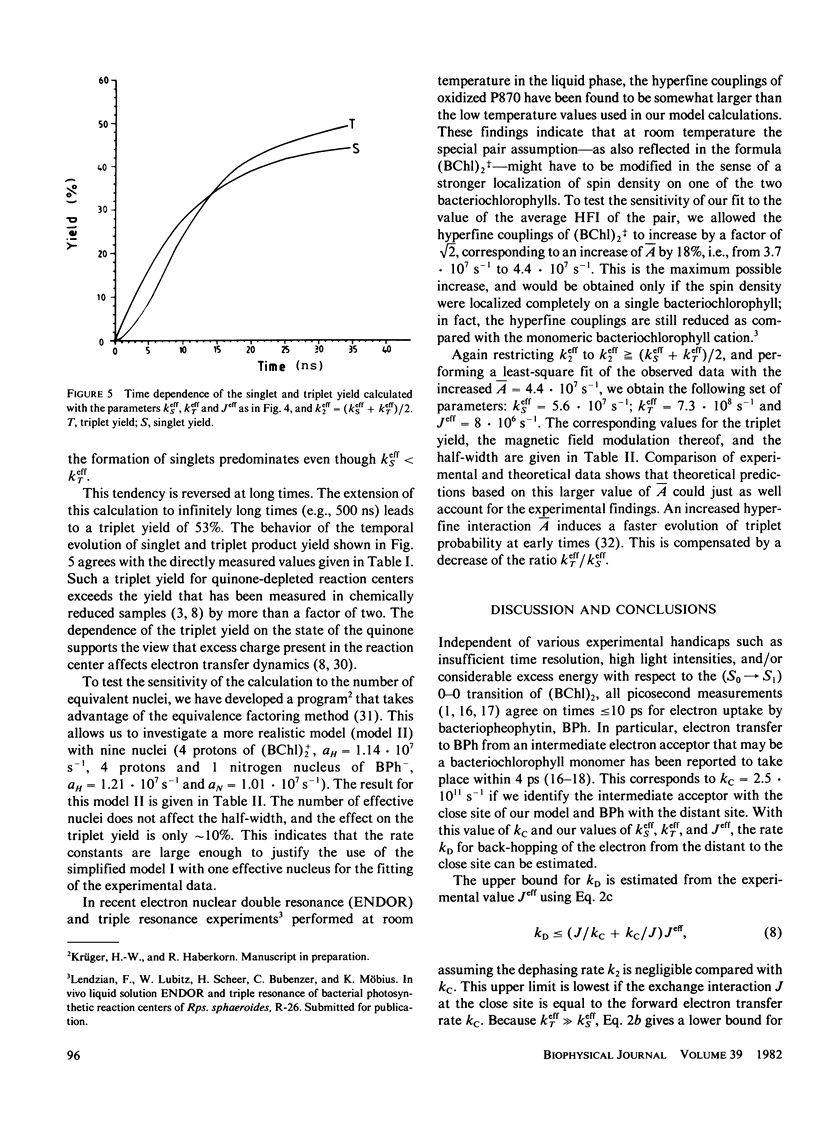
The time dependence of magnetic field effects on light absorption by triplet-state and radical ions in quinone-depleted reaction centers of Rhodopseudomonas sphaeroides strain R-26 has been investigated. Measurements on the time scale of the hyperfine interaction in the radical pair [(BChl)2+. ...BPh-.)] provided kinetic data characterizing the recombination process. The results have been interpreted in terms of a recently proposed model that assumes an intermediate electron acceptor (close site) between the bacteriochlorophyll "special pair" (BChl)2 and the bacteriopheophytin BPh (distant site). Recombination is assumed to proceed through this intermediate acceptor. The experiments led to effective recombination rates for the singlet and triplet channel: k(Seff) = 3.9 . 107 s-1 and k(Teff) = 7.4 . 10(8) s-1. These correspond to recombination rates ks = 1 . 10(1) s-1 and kT = 7.1 . 10(11) s-1 in the close configuration. The upper bound of the effective spin dephasing rate k2eff approximately equal to 1 . 10(9) s-1 is identical with the rate of the electron hopping between the distant site of zero spin exchange interaction and the close site of large interaction. Interpretation of data for the case of direct recombination yields the recombination rates, spin dephasing rate, and exchange interaction in a straightforward way.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship R. E., Schaafsma T. J., Parson W. W. Magnetic field effects on radical pair intermediates in bacterial photosynthesis. Biochim Biophys Acta. 1977 Aug 10;461(2):297–305. doi: 10.1016/0005-2728(77)90179-7. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Leigh J. S., Jr, Reed D. W. Primary events in the photosynthetic reaction centre from Rhodopseudomonas spheroides strain R26: triplet and oxidized states of bacteriochlorophyll and the identification of the primary electron acceptor. Biochim Biophys Acta. 1973 Apr 5;292(3):654–664. doi: 10.1016/0005-2728(73)90013-3. [DOI] [PubMed] [Google Scholar]
- Fajer J., Brune D. C., Davis M. S., Forman A., Spaulding L. D. Primary charge separation in bacterial photosynthesis: oxidized chlorophylls and reduced pheophytin. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4956–4960. doi: 10.1073/pnas.72.12.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkorn R., Michel-Beyerle M. E., Marcus R. A. On spin-exchange and electron-transfer rates in bacterial photosynthesis. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4185–4188. doi: 10.1073/pnas.76.9.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberkorn R., Michel-Beyerle M. E. On the mechanism of magnetic field effects in bacterial photosynthesis. Biophys J. 1979 Jun;26(3):489–498. doi: 10.1016/S0006-3495(79)85266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff A. J., Rademaker H., van Grondelle R., Duysens L. N. On the magnetic field dependence of the yield of the triplet state in reaction centers of photosynthetic bacteria. Biochim Biophys Acta. 1977 Jun 9;460(3):547–554. doi: 10.1016/0005-2728(77)90094-9. [DOI] [PubMed] [Google Scholar]
- Holten D., Hoganson C., Windsor M. W., Schenck G. C., Parson W. W., Migus A., Fork R. L., Shank C. V. Subpicosecond and picosecond studies of electron transfer intermediates in Rhodopseudomonas sphaeroides reaction centers. Biochim Biophys Acta. 1980 Oct 3;592(3):461–477. doi: 10.1016/0005-2728(80)90092-4. [DOI] [PubMed] [Google Scholar]
- Holten D., Windsor M. W. Picosecond flash photolysis in biology and biophysics. Annu Rev Biophys Bioeng. 1978;7:189–227. doi: 10.1146/annurev.bb.07.060178.001201. [DOI] [PubMed] [Google Scholar]
- Jortner J. Dynamics of electron transfer in bacterial photosynthesis. Biochim Biophys Acta. 1980 Dec;594(4):193–230. doi: 10.1016/0304-4173(80)90001-4. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Petty K. M., Dutton P. L., Rentzepis P. M. Picosecond kinetics in reaction centers of Rps. sphaeroides and the effects of ubiquinone extraction and reconstitution. Biochem Biophys Res Commun. 1976 Jun 7;70(3):839–845. doi: 10.1016/0006-291x(76)90668-9. [DOI] [PubMed] [Google Scholar]
- Michel-Beyerle M. E., Scheer H., Seidlitz H., Tempus D., Haberkorn R. Time-resolved magnetic field effect on triplet formation in photosynthetic reaction centers of Rhodopseudomonas sphaeroides R-26. FEBS Lett. 1979 Apr 1;100(1):9–12. doi: 10.1016/0014-5793(79)81120-5. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Scheer H., Katz J. J. Models for antenna and reaction center chlorophylls. Ann N Y Acad Sci. 1975 Apr 15;244:260–280. doi: 10.1111/j.1749-6632.1975.tb41535.x. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillotin G., Vermeglio A., Breton J. Orientation of reaction center and antenna chromophores in the photosynthetic membrane of Rhodopseudomonas viridis. Biochim Biophys Acta. 1979 Feb 8;545(2):249–264. doi: 10.1016/0005-2728(79)90204-4. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Cogdell R. J. The primary photochemical reaction to bacterial photosynthesis. Biochim Biophys Acta. 1975 Mar 31;416(1):105–149. doi: 10.1016/0304-4173(75)90014-2. [DOI] [PubMed] [Google Scholar]
- Pellin M. J., Wraight C. A., Kaufmann K. J. Modulation of the primary electron transfer rate in photosynthetic reaction centers by reduction of a secondary acceptor. Biophys J. 1978 Oct;24(1):361–369. doi: 10.1016/S0006-3495(78)85383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker H., Hoff A. J. The balance between primary forward and back reactions in bacterial photosynthesis. Biophys J. 1981 May;34(2):325–344. doi: 10.1016/S0006-3495(81)84852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvalov V. A., Asadov A. A. Arrangement and interaction of pigment molecules in reaction centers of Rhodopseudomonas viridis. Photodichroism and circular dichroism of reaction centers at 100 k. Biochim Biophys Acta. 1979 Feb 8;545(2):296–308. doi: 10.1016/0005-2728(79)90207-x. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Parson W. W. Energies and kinetics of radical pairs involving bacteriochlorophyll and bacteriopheophytin in bacterial reaction centers. Proc Natl Acad Sci U S A. 1981 Feb;78(2):957–961. doi: 10.1073/pnas.78.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnauer M. C., Katz J. J., Norris J. R. The triplet state in bacterial photosynthesis: Possible mechanisms of the primary photo-act. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3270–3274. doi: 10.1073/pnas.72.9.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voznyak V. M., Elfimov E. I., Sukovatitzina V. K. Magnetic field affects the fluorescence yield in reaction center preparations from Rhodopseudomonas spaeroides R-26. Biochim Biophys Acta. 1980 Sep 5;592(2):235–239. doi: 10.1016/0005-2728(80)90184-x. [DOI] [PubMed] [Google Scholar]
- Werner H. J., Schulten K., Weller A. Electron transfer and spin exchange contributing to the magnetic field dependence of the primary photochemical reaction of bacterial photosynthesis. Biochim Biophys Acta. 1978 May 10;502(2):255–268. doi: 10.1016/0005-2728(78)90047-6. [DOI] [PubMed] [Google Scholar]


